Pollinator and pollination mechanism of Impatiens furcillata (Balsaminaceae) in Korea
Article information
Abstract
An effective pollinator was investigated based on visiting insects to confirm the pollination mechanism of Impatiens furcillata Hemsl. (cheo-jin-mul-bong-seon), an annual herb that is also a species endemic to Korea that has hardly been studied in relation to pollination ecology. The insects that visited the group of I. furcillata studied here consisted of four orders, 11 families, and 16 species; Hymenoptera had seven species (43.8%), Lepidoptera had four (25.0%), Diptera four (25.0%), and Hemiptera one (6.2%). Visiting insects were divided into those that took only nectar, those that took nectar and pollen, and those that took neither. Insects that are effective for pollination are judged considering the length and body type of their mouth parts, and Amegilla florea Smith (huin-jul-beol) is judged to be the most effective pollinator in the survey area. As a result of observing pollination behavior, when visiting a flower, A. florea, which extended its glossa, approached the front, landed on a wing petal of I. furcillata, crawled into the flower tube, and then backed up and reversed its steps, with pollen adhered to its back. The findings here present basic information about species biology related to both I. furcillata and A. florea.
INTRODUCTION
Approximately 850 species of Balsaminaceae are distributed worldwide (Gery-Wilson, 1980; Chen et al., 2007), and the three taxa Impatiens textorii Miq., I. noli-tangere L., and I. furcillata Hemsl. exist in Korea (Oh, 2007). Impatiens is morphologically classified according to the color and shape of the flower, the shape of the spur, and the shape of the leaf. Impatiens furcillata is distinguished by the characteristics of a small flower, a light pink color, and by the fact that it droops downward without curling (Ji et al., 2010).
Impatiens furcillata is a floristic special plant class IV and a vulnerable species (VU) on the IUCN Red List. It is also an endemic species in Korea, distributed on Jangdo Island and Gageodo Island in Sinan-gun (Son et al., 2013) and Mt. Dalmasan in Haenam-gun (Lee et al., 2009), Mt. Cheongwansan, in Jangheung-gun (Lim and Im, 2002), on Mt. Bongnaesan in Goheung-gun, on Mt. Byeokbangsan in Tongyeong-si (Oh et al., 2016) and Mt. Geumosan in Hadong-gun. It is a native plant that is locally distributed on the south coast and the southwestern islands of Korea and is important taxonomically and biologically regarding conservation due to the small size of each group. It is also a genetic resource with a high potential for development into horticultural varieties, considering the shape of its flowers and its growth characteristics.
The seeds of Impatiens are produced through both chasmogamy and cleistogamy in what is interpreted as a mixed reproductive strategy. This gives Impatiens a relatively high reproductive success rate in response to various growth environments (Harper, 1997; Lloyd, 1984). Impatiens plants also have the advantage of being able to secure a certain amount of seeds through self-pollination using chasmogamy if reproduction by cleistogamy fails for reasons such as pollinator issues, resource limitations, and low levels of photosynthesis (Hong, 2001). Chasmogamy is an important breeding strategy as an auxiliary and complementary means of reproduction, but cross-pollination by pollinators is a key breeding strategy that enables continuous species maintenance and evolution by increasing the genetic diversity of natural populations. Therefore, the relationship with pollinators is an important factor in determining the reproductive success rate, distribution area, and genetic variation and segmentation of plants (Blancafort and Gómez, 2005, Kim and Park, 2014).
In the general structure of a flower, the location of the pollen is such that insects can easily access it, and insect mouthparts are unrelated to this (Knuth, 1906–1909). Moreover, the distance from the entrance of the flower to the nectar gland requires morphological and structural specificity, akin to the relationship between a lock and a key (Jolivet, 1992; Son, 2010). The flowers of Impatiens attract various insects because they are large and showy and produce a large quantity of nectar. Because nectar is secreted from the middle to the tip of the long-developed spur at the rear of the flower, it is known that insects that obtain nectar have a body shape suitable for the structure of the flower with specificity as pollinators.
Recently, studies of the pollination ecology of Impatiens species have been active (Kato, 1988; Tian et al., 2004; Janecek et al., 2015; Tokuda et al., 2015; Ruchisansakun et al., 2016). In Cameroon, a bird, Cyanomitra oritis, is an important pollinator of all Impatiens species (Janecek et al., 2015). A study of the floral variation and pollination of seven co-occurring Impatiens spp. in a Southeast Asian diversity hotspot reveals that typically several animals visit these plants, but the most effective pollinator differs depending on the structure of the flower (Ruchisansakun et al., 2016). In this context, the means of cross-pollination can also facilitate the reproduction of I. reptans Hook. f. depending on a specialized habitat, a narrow environmental niche, a low percentage of seed germination, and habitat loss (Tian et al., 2004). One study found that in Japan, I. textori and I. noli-tangere are competing pollinators in the wild (Tokuda et al., 2015). According to one study that investigated characteristic patterns of behaviors in flower use by insects, Bombus diversus was found to be a visitor of three species in Japan (Kato, 1988). However, researchers have not investigated effective pollinators of I. furcillata. Therefore, in this study, field investigations of the flowering process and visiting insects were conducted to identify effective pollinators and the pollination mechanisms of I. furcillata. These results are expected to provide basic ecological data for researchers of the life cycle of I. furcillata.
MATERIALS AND METHODS
A population of I. furcillata in the northern slope of Mt. Cheongwan in Jangheung-gun, Jeollanam-do is investigated. The habitat of I. furcillata on Mt. Cheongwan is vacant, stony ground in the Torreya nucifera community, where a canopy had not formed, and it is a sloping frontier site with an inclination angle of 30°. Impatiens furcillata in the observed group germinates in March–April, first blooms in July, blooms until September, and repeats the life cycle of seed formation and death until early October. In order to investigate the insects that visit I. furcillata, they were observed once a week from 9:00 am to 5:00 pm during the flowering period from July to September 2021 and from July to September 2022. The types of visiting insects and the number of visits were recorded. Insects were collected for accurate species identification. In order to identify effective insects that mediate pollination based on the morphological characteristics of flowers, 50 flowers were collected from the observation group and the shape and size (long axis; length of the side of the flower, short axis; length of the front side of the flower) were measured. In addition, the possibility as a pollinator was confirmed by checking whether pollen was attached to the visiting insects.
RESULTS AND DISCUSSION
Flower structure and pollination
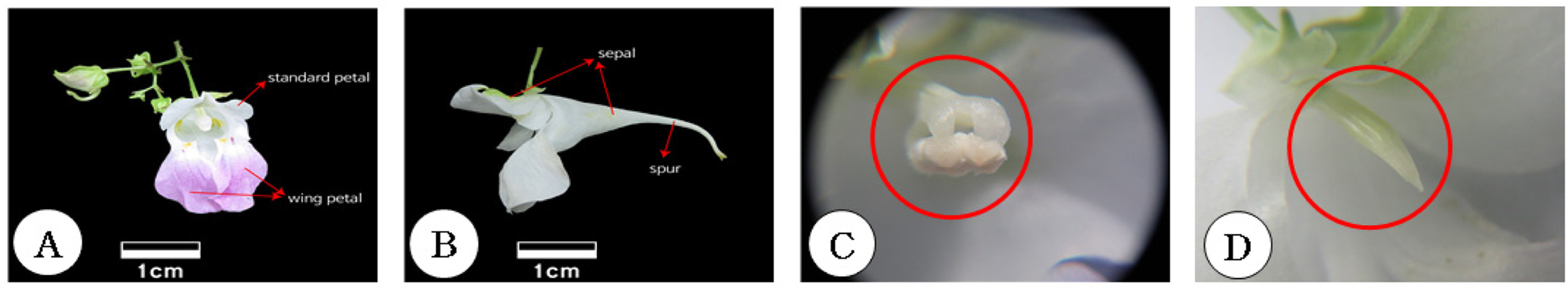
Impatiens furcillata has racemes with one to five pale pink flowers close to white on a peduncle that hangs down in an axil. The petals are round, and there is one pink standard petal with a keel-like protrusion on the back main vein and two wing petal split into two lobes (Fig. 1A). The calyx is composed of two green calyxes attached to the peduncle and the flower tube including the spur, with the nectary found between the middle and the end of the spur (Fig. 1B). There are five stamens, and the pollinia firmly attached to the filament are combined with each other and are bent toward the inside of the flower tube. One pistil is buried at the bottom of the pollinium (Fig. 1C). Pollen is transferred to the body of the insect when it enters and exits the inside of the flower to take nectar. When the pollen source is exhausted due to frequent visits by insects, the stigma is exposed, and the pollen adhering to the body of the insect is transferred to the stigma, at which point cross-pollination occurs, pollination is terminated, and the petals eventually fall (Fig. 1D)
Visiting insects
Insects that visited I. furcillata during the survey period consisted of four orders, eleven families, and 16 species (Table 1). Among the visiting insects, there are a total of seven species of Hymenoptera: Amegilla florea, Apis mellifera, Xylocopa appendiculata circumvolans, Sphecodes pallidulus, Vespula koreensis koreensis, Vespa mandarinia, and Taeniogonalos fasciata. One species of Hemiptera, Bothrogonia ferruginea, was identified. The four species of Lepidoptera identified were Papilio protenor, Macroglossum bombylans, Eurema mandarina, and Pieris rapae, and the four species of Diptera found were Mallota dimorpha, Episyrphus balteatus, Drinomyia hokkaidensis, and Sarcophaga melanura. Visiting insects were grouped into the three types of insects that only take nectar, insects that take nectar and pollen, and insects that take neither.
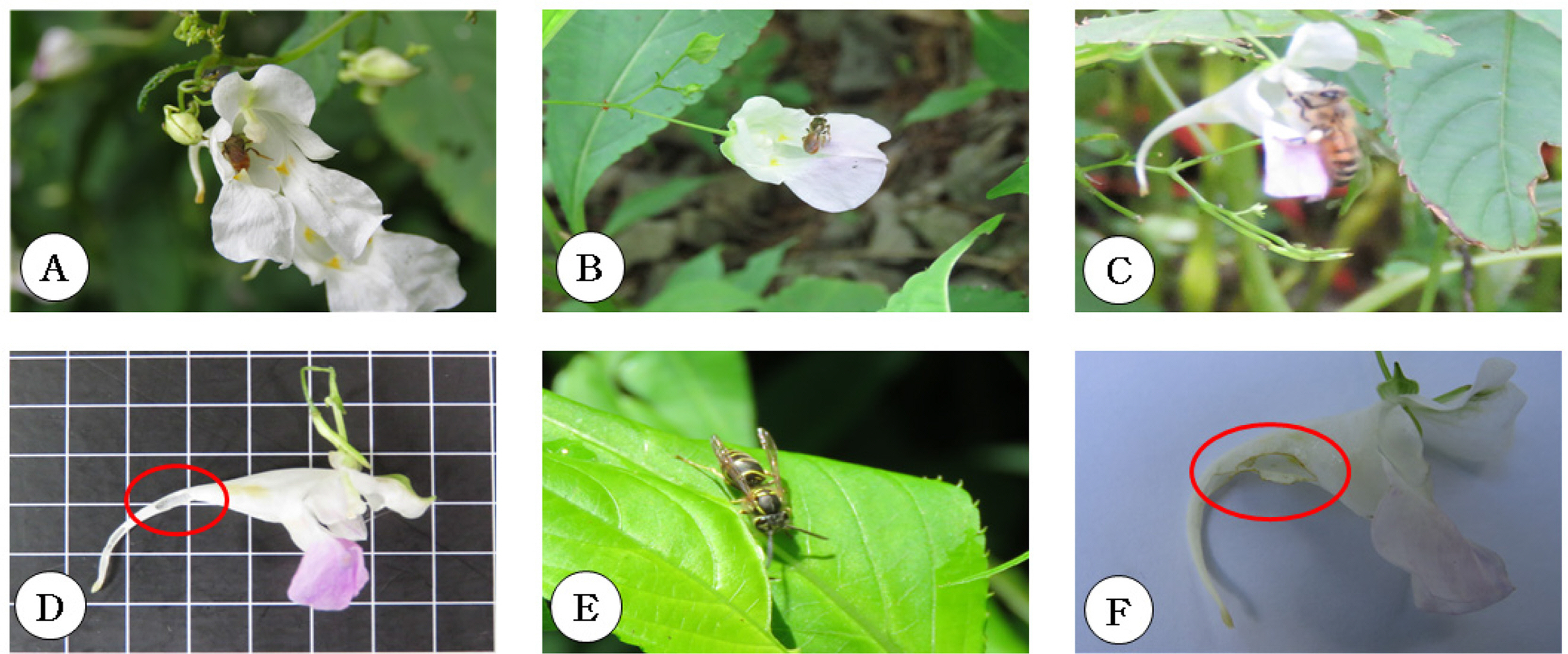
Insects that only take nectar: Among the insects that visited I. furcillata, six species were identified as only nectartaking insects: Amegilla florea, Xylocopa appendiculata circumvolans, Papilio protenor, Macroglossum bombylans, Eurema mandarina, and Pieris rapae (Fig. 2). Amegilla florea and Xylocopa appendiculata circumvolans may be effective insects for pollination by entering the flower tube and taking nectar. However, the body size of Xylocopa appendiculata circumvolans (12–14 mm) exceeds the flower height (8–10 mm), meaning that Xylocopa appendiculata circumvolans is not a suitable pollinator, as its crawling behavior damages the flower. In the four species belonging to Lepidoptera, the insects readily ate nectar with their mouthparts, reaching through the interior of the flower to the nectar-bearing part. However, it was found that these are not effective pollinators because they did not touch the pollinium when they took nectar and pollen was not detected on their bodies after doing so.

Insects that only take nectar. A, B. Amegilla florea (A, landing on flowers; B, going inside a flower and only taking nectar). C. Papilio protenor (taking only nectar using its long proboscis). D. Xylocopa circumvolans (going inside a flower and taking only nectar). E. Macroglossum bombylans (taking only nectar using its long proboscis). F. Pieris rapae (taking only nectar using its long proboscis).
Insects that take nectar and pollen: Among the 16 insect species that visited I. furcillata, three were identified as those that took both nectar and pollen: Sphecodes pallidulus, Apis mellifera, and Vespula koreensis koreensis (Fig. 3). Sphecodes pallidulus entered the middle of the spur and ingested nectar, but it was found that pollen did not reach the head and back of Sphecodes pallidulus due to its low body height (2–3 mm). In addition, Apis mellifera and Vespula koreensis koreensis were observed to take nectar by collecting pollen or damaging the spur from the outside of the flower. From these behavioral observations, it was found that Sphecodes pallidulus, Apis mellifera, and Vespula koreensis koreensis did not act as pollinators for I. furcillata.
Insects that take nectar and pollen. A, B. Sphecodes pallidulus (A, going inside a flower and taking only nectar; B, taking pollen and resting). C, D. Apis mellifera (C, taking pollen while holding the pistil and stamens; D, damaging the nectary). E, F. Vespula koreensis koreensis (E, taking nectar and resting; F, damaging the nectary).
Simply visiting insects There were seven species of simple visiting insects: Vespa mandarinia, Taeniogonalos fasciata, Bothrogonia ferruginea, Mallota dimorpha, Episyrphus balteatus, Drinomyia hokkaidensis, and Sarcophaga melanura. It was found that simply visiting insects landed on the leaves or flowers of I. furcillata but did not consume nectar or come in to contact with pollen and did not thus participate in pollination.
Pollinator and pollination behavior
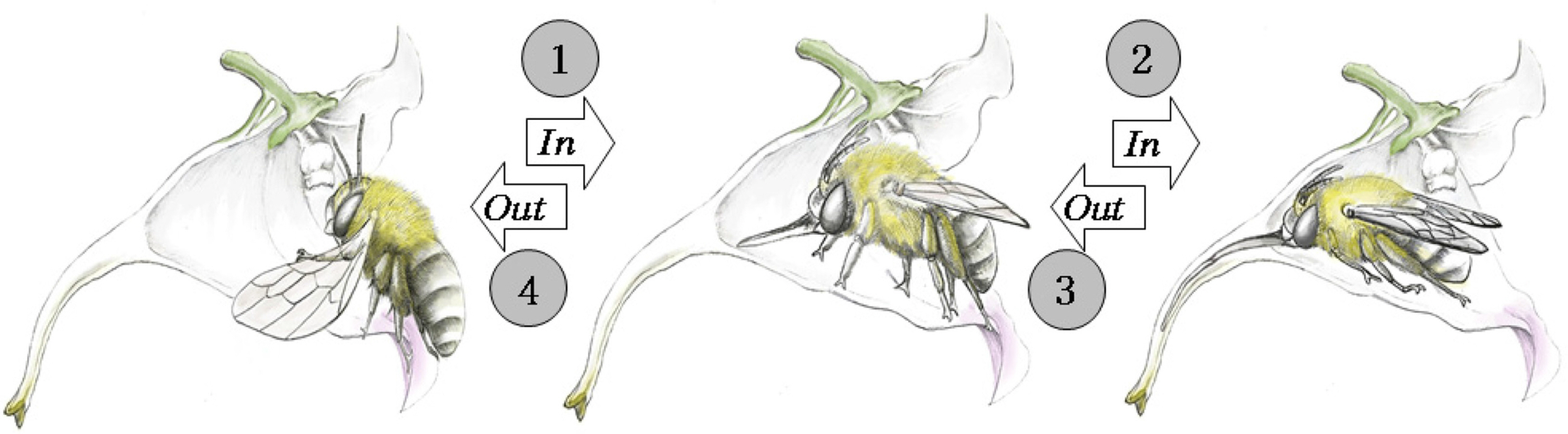
As a result of observing the pollination behavior of A. florea visiting I. furcillata during the investigation period, the following behaviors were found. When A. florea visits a flower, it approaches the flower from the front and sits holding the wing petals with its legs. It crawls into the flower tube, extends its glossa, takes the nectar, and then walks backwards. At this time, the pollen touches the back of A. florea, and it leaves the flower with pollen on the hairs on the back (Fig. 4).
The pistil of I. furcillata is wrapped in five stamens that form a lump and hang from a standard petal (Fig. 5A). Because the height of the pollen and wing petals is 6–9 mm, pollen can be deposited if the height of the visiting insect’s back exceeds 6 mm. In addition, the nectar location of I. furcillata is distributed from the middle to the end of the spur (Fig. 5B). In order to ingest nectar, only an insect with a body length of at least 20 mm, including the extended glossa, can serve as an effective pollinator. The body length of A. florea is about 15 mm the length when extending its glossa is approximately 26 mm, and its body height is 6–9 mm thus exceeding 6 mm (Fig. 5C).

Flowers and pollinators. A. Flower of Impatiens furcillata. B. Location of floral nectar as indicated by the red circle. C. glossa extended. D. Pollen from I. furcillata attached to the back of Amegilla florea, as indicated by the red circle.
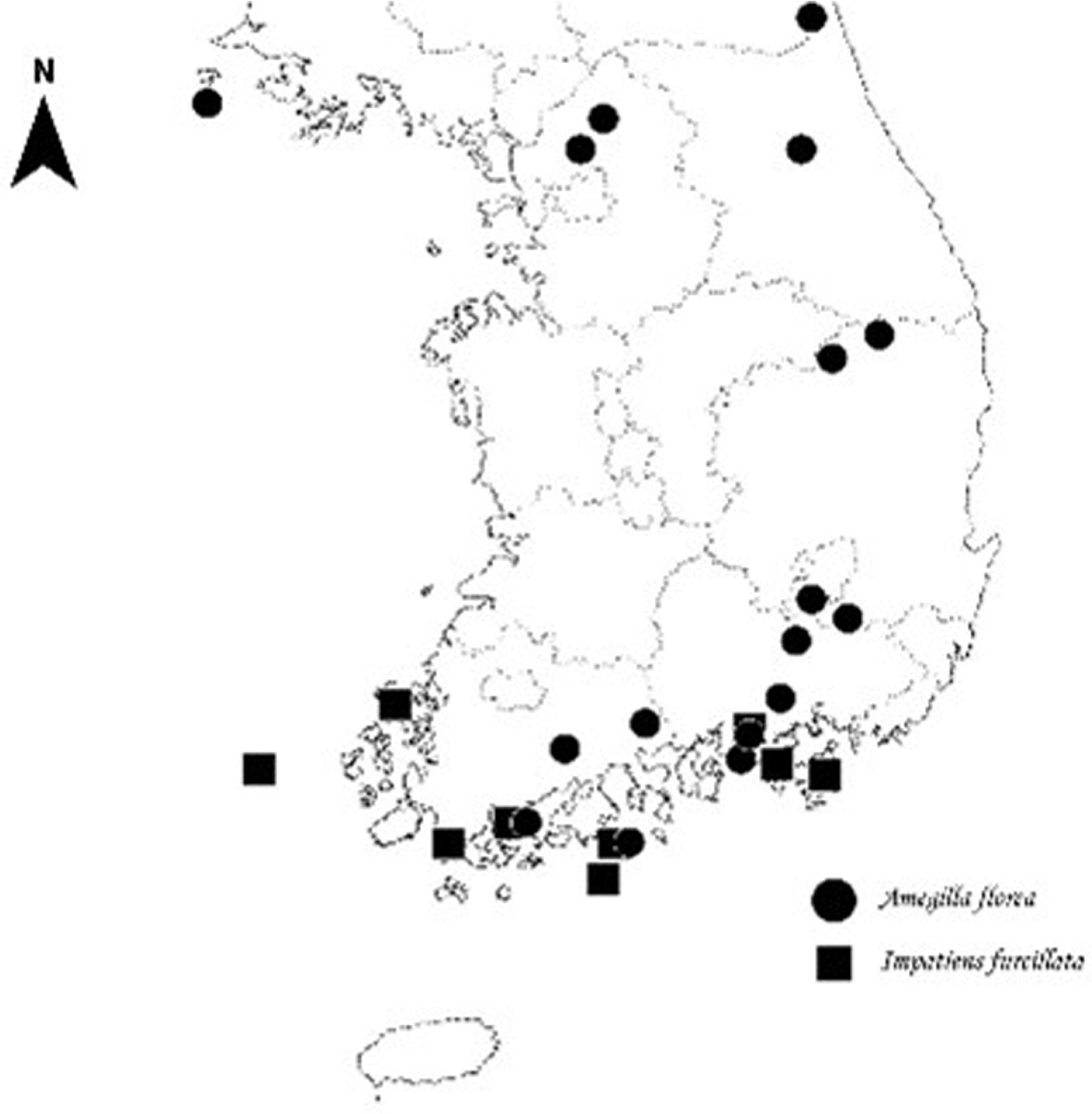
Considering the flower shape of I. furcillata and the location of nectar distribution, A. florea has the characteristics required to be a sufficient pollinating insect of I. furcillata. That is, the mouthpart is 10 mm or more, the body length including the mouthpart is at least 20 mm or more, and the body height is 6 mm or more but less than 10 mm. In addition, there are hairs onto which pollen can become attached to the back or head of A. florea when it exits backward, and pollen of I. furcillata even confirmed to exist on the back of A. florea after it visits the flower (Fig. 5D). During the research period, the average number of visits to A. florea amounted to 32, greater than that of other insects, and the number of visits to I. furcillata per survey period was 20 to 30, indicating that more individuals than other insects visited. For these reasons, the most effective pollinator of I. furcillata in this study region is presumed to be A. florea. The fact that A. florea is distributed in the survey area through specimen verification also supports this contention (Fig. 6).
Through the results of investigating effective pollinators and their pollination mechanisms in relation to I. furcillata on Mt. Cheongwansan in Jangheung-gun, A. florea is revealed to act as an effective pollinator because it has the most effective morphological characteristics, in this case its body height, body length, and mouthpart length when extending its glossa. It also has the required behavioral characteristics related to florae. Unlike Cameroon, for which a bird acts as a pollinator of Impatiens, I. furcillata in Korea it is a bee that is an effective pollinator (Janecek et al., 2015). A reason behind this pollinator difference can be found through a study that investigates floral variation and pollination of seven co-occurring Impatiens spp. types in Southeast Asia (Ruchisansakun et al., 2016). In addition, future research could focus on pollinator competition among Impatiens spp. types in Korea depending on a specialized habitat, a narrow environmental niche, a low percentage of seed germination, and habitat loss (Tian et al., 2004; Tokuda et al., 2015). Detailed studies related to flower visitation patterns and efficiency in relation to I. furcillata can also provide more detailed information about pollination mechanisms.
Acknowledgements
We thank Haeng-Jung Kim for drawing a picture of the pollination process of A. florea Smith.
Notes
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.