Tectariaceae Panigrahi was accepted as an independent family in a revision of fern classification in 2006 (Panigrahi, 1986; Smith et al., 2006). The family Tectariaceae formed a large clade with Oleandraceae, Polypodiaceae, and Davalliaceae. It is the basal lineage among the four families of the clade (Smith et al., 2006). Tectariaceae has usually short-creeping to ascending rhizomes with scales. The fronds are monomorphic to strongly dimorphic. The blades are pinnate, bipinnate, or decompound. The rachis and costae are usually covered by articulate multicellular hairs. The indusia are reniform or peltate. The spores are of a brownish color and are monolete or monolete with various ornaments. (Smith et al., 2006; Sun et al., 2014). Within Tectariaceae, Tectaria is the largest genus and it is taxonomically confused (Cavanilles, 1799; Copeland, 1947). This genus contains 150–210 species that are mostly distributed in tropical areas (Tryon and Tryon, 1982; Holttum, 1991). Most members of the genus Tectaria have unlobed basal pinnae, but in some species the basal pinnae are lobed, in which case the basal basiscopic lobes or pinnule are the largest. The veins are copiously anastomosing, although some species have free veinlets (Sun et al., 2014). Since Tectaria has been generally accepted as a distinct genus (Underwood, 1906; Copeland, 1907; Ching, 1931; Christensen, 1934), its taxonomic delimitation has proved controversial (Ding et al., 2014). Molecular studies on plastid and nuclear markers of Tectaria strongly supported the monophyly (Ding et al., 2014; Zhang et al., 2016; Zhang et al., 2017). The most recent molecular data suggest that Tectaria is composed of four superclades and 12 major clades (Zhang et al., 2017).
Here, Tectaria fuscipes (Wall. ex Bedd.) C. Chr. of the family Tectariaceae is newly reported from Korea (Figs. 1, 2). The local name of the family and genus were given as “Mi-neul-chang-go-sa-ri” based on the English common name of “Halberd fern”. In botanical terminology, “Halberd” means arrowhead shaped with the basal lobes turned outward. Many Tectaria species have a halberd-shaped blade or pinna at the frond apex. “Halberd” is translated as “Mi-neul-chang” in Korean. “Mi-neul-chang” means a spear with two or three-pronged strands ending like branches and “Go-sa-ri” means fern. The Korean name of T. fuscipes, “Baek-rok-go-sa-ri”, means the white snow-covered slope where it was first discovered.
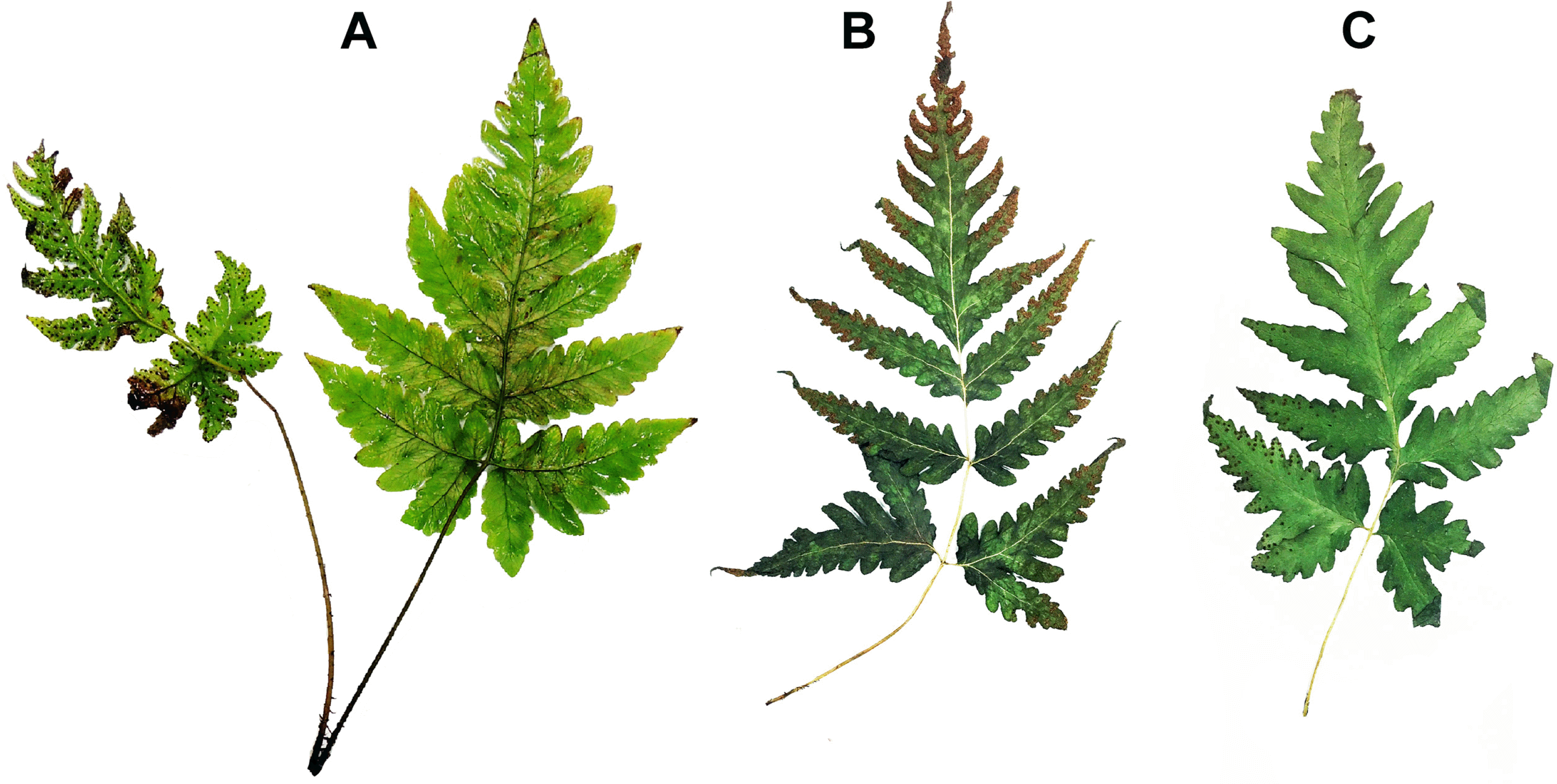
According to the molecular phylogeny of the genus Tectaria, T. fuscipes is included in the Ctenitopsis superclade and Ctenitopsis major clade (Zhang et al., 2017). Tectaria fuscipes has previously been treated as a synonym of T. paradoxa (Fée) Sledge (Sledge, 1972). On the other hand, Patil et al. (2014) distinguished T. fuscipes from T. paradoxa on account of its dimorphic fronds. However, partially monomorphic fronds were found in the population of Jeju Island, as in other studies (Wu et al., 2013; Sun et al., 2014) (Fig. 3). According to the frond type, T. fugipes of Jeju should be T. paradoxa, but the 13 commonly used morphological characters were all revealed as homoplastic in Tectaria in the recent phylogenetic studies (Ding et al., 2014; Zhang et al., 2017). This means that frond type is not a key character to distinguish T. fuscipes and T. paradoxa. The isotype specimen of Aspidium paradoxum (Herbier de l’Universite Montpellier II (MPU), MPU015614), which represents the basionym of T. paradoxa, differs morphologically from specimens of T. fuscipes collected on Jeju Island. Also, recent studies still maintain this synonymous name with insufficient information (Ding et al., 2014; Patil et al., 2014; Zhang et al., 2016; Zhang et al., 2017). Therefore, we use the name T. fugipes instead of T. paradoxa.
Several species of Tectaria have been reported from southern China (Guangxi, Guizhou, Hainan, Taiwan, Xizang, and Yunnan) and eight species have been reported in Japan (Iwatsuki et al., 1995; Wu et al., 2013). However, to date T. fuscipes has not been recorded in Japan. This species is also reported in India and most Southeast Asian countries: Cambodia, Indonesia, Myanmar, Nepal, Taiwan, Thailand, Vietnam (Ching, 1931; Holttum, 1988; Wu et al., 2013; Patil et al., 2014; Sun et al., 2014). In previous studies, the easternmost and northernmost distribution of the species was known to be Taiwan and Xizang, respectively. The new discovery on Jeju Island therefore represents a northeastern extension of its range, and suggests that T. fuscipes may occur in the southern part of Japan. Additionally, other species of Tectariaceae which are native to China, Japan, and Taiwan may yet be found in Korea. Tectaria fuscipes grows in full sun 3–4 m from the entrance of a small, humid cave. This cave is situated at 300–400 m above sea level on the southern slopes of Mt. Halla, and there is a distinct temperature difference across its entrance. This specific habitat is very limited, making T. fuscipes prone to disturbance and extirpation. Only ca. 50 individuals were seen in an area of approximately 3 m2. Careful research needs to be conducted to survey more suitable habitats of T. fuscipes and to conserve this rare species in Korea.
Taxonomic Treatment
Tectariaceae Panigrahi
Pantropical terrestrial herbs. Rhizome usually short-creeping to ascending, dictyostelic, bearing scales; petioles not abscising, with a ring of vascular bundles in cross-section; blades simple, pinnate, or bipinnate, sometimes decompound; indument of jointed, usually short stubby hairs on the axes, veins, and sometimes laminar tissue, especially on rachises and costae adaxially; veins free or often highly anastomosing, sometimes with included veinlets; indusia reniform or peltate (lost in several lineages); spores brownish, reniform, monolete, variously ornamented; x = 40 (a few genera with x = 41, some dysploids with x = 39).
Selected reference: Smith, A. R., K. M. Pryer, E. Schuettpelz, P. Korall, H. Schneider, and P. G. Wolf. Taxon 55: 705–731, 2006.
Korean name: Mi-neul-chang-go-sa-ri-gwa (미늘창고사리 과) (신칭).
Tectaria Cav.
Plants terrestrial or lithophytic. Stems short-creeping to erect, stolons absent. Leaves monomorphic, evergreen. Petiole 3/4–3 times length of blade, base not swollen; vascular bundles more than 3, arranged in an arc, ±round in cross section. Blade lanceolate to deltate or pentagonal, entire to 1-pinnate-pinnatifid [3-pinnate-pinnatifid], reduced distally to shallowly lobed or hastate apex, herbaceous to papery. Pinnae not articulate to rachis, segment margins entire to sinuate or shallowly lobed; proximal pinnae not or only slightly reduced, sessile to short-petiolulate, base equilateral or often inequilateral with prominent basiscopic lobe(s); costae adaxially rounded or shallowly grooved, grooves not continuous from rachis to costae; indument lacking or of multicellular hairs on costae abaxially, of multicellular hairs on costae adaxially. Veins reticulate, areoles with or without included veinlets. Sori in 1–several rows between midrib and margin, round; indusia peltate to round-reniform and with narrow sinus, persistent or caducous. Spores brownish, with inflated folds or wings. x = 40.
Selected reference: Morton, C. V. 1966. Am. Fern J. 56: 120–137, 1966.
Korean name: Mi-neul-chang-go-sa-ri-sok (미늘창고사리 속) (신칭).
Tectaria fuscipes (Wall. ex Bedd.) C. Chr., Contr. U. S. Natl. Herb. 26: 290, 1931; Aspidium fuscipes Wall. ex Bedd., Suppl. Ferns Brit. Ind. 15, pl. 366, 1876; Lastrea fuscipes (Wall. ex Bedd.) Bedd., Handb. Ferns Brit. India. 243, 1883; Nephrodium fuscipes (Wall. ex Bedd.) C. B. Clarke, Symb. Sin. 6: 24, 1929; Ctenitopsis fuscipes (Wall. ex Bedd.) C. Chr., Notul. Syst. (Paris) 7: 87, 1938.
Nephrodium membranifolium var. dimorpha C. B. Clarke, Trans. Linn. Soc. Lond., Bot. 1: 535, pl. 75, f. B C, 1880.
Pleocnemia membranifolia auct. non C. Presl: Bedd., Handb. Ferns Brit. India: 225, f. 115, 1883.
Aspidium membranifolium var. dimorphum (C.B. Clarke) Christ, J. Bot. (Morot) 19: 4, 62, 1905.
Ctenitopsis glabra Ching & C. H. Wang, Acta Phytotax. Sin. 9: 370, 1964.
Korean name: Baek-rok-go-sa-ri (백록고사리).
Plant to 50 cm tall. Rhizome short, suberect to ascending; scales stiff, glossy, lanceolate, dark brownish or blackish, ca. 7 mm long. Fronds clustered, dimorphic or subdimorphic, fertile fronds narrower. Stipe ca. 15 cm long, deep stramineous or brownish, sparsely scaly, densely hairy; scales linear, ca. 2 mm long. Blade pinnate or 2-pinnatifid to 2-pinnate, oblong-lanceolate or narrowly deltoid, 20–35 cm long, 10–20 cm wide, herbaceous, acuminate at apex, hairy throughout; pinnae 5–8 pairs; rachises and costae with sparse linear scales and densely hairs; basal pair of pinnae largest, more lobed, asymmetrically oblong-subdeltoid, stalks ca. 1 cm; middle pinnae lanceolate or oblong-lanceolate, 6–10 cm long, 2– 4 cm wide, base truncate or slightly cordate, apex acute to acuminate or caudate, lobed 1/3–1/2 to costa; bearing large basal basiscopic pinnules, 4–6 cm long, 1.5–2.5 cm wide, pinnatifid or pinnatipartite. Veins free, pinnate in ultimate segments, veinlets simple or occasionally forked, reaching margin. Sori terminal on acroscopic branches of veinlets, medial in ultimate segments or rather irregularly dispersed on lower surface of pinnae; indusia round-reniform, hairy, persistent.
Voucher specimens: KOREA. Jeju-do: Seogwipo-si, Mt. Hallasan, alt. 320 m, 7 Mar 2017, M. J. Kim, C. K. Oh, N. S. Lee, & J. H. So (2 sheets) (EWH); Seogwipo-si, Mt. Hallasan, alt. 320 m, 7 Sep 2017, M. J. Kim (3 sheets) (EWH).
Collection site: Pit cave on the southwestern slope of Mt. Hallasan (alt. 320 m), Seogwipo-si, Jeju-do, Korea.
Habitat: Tectaria fuscipes grows in sun close to the entrance of a cave. The cave has a lot of moisture and the width of the cave opening is about 3 m. Tectaria fuscipes grows with Arachniodes rhomboidea and Crepidomanes sp. The vegetation in the vicinity consists of coppiced Mallotus japonicus, Rosa multiflora, Smilax china, etc.
Distribution: Cambodia, south China (Guangxi, Guizhou, Hainan, Xizang, and Yunnan), India (north east India including Assam and Sikkim and Western Ghats, Castle Rock), Indonesia (east Java and southwest Sulawesi), south Korea (Jeju Island), Myanmar, Nepal, Taiwan, Thailand, and Vietnam.