INTRODUCTION
Menyanthaceae is a family of aquatic plants in the order Asterales (Angiosperm Phylogeny Group, 2016). There are 76 species in the six genera of, Liparophyllum Hook.f., Nymphoides Ség., Menyanthes L., Ornduffia Tippery & Les, Nephrophyllidium (Menz. ex Hook.) Gilg., and Villarsia Vent. Among them, Menyanthes is a monotypic genus. This species is distributed in the Northern Hemisphere, specifically Asia and Europe (Hewett, 1964). It is also a rare and endangered aquatic plant in Korea (Korea National Arboretum, 2021). The basic chromosome number in Menyanthes is x = 9 (Peruzzi and Cesca, 2004). After being described in Species Plantarum (Linnaeus, 1753), the family was classified as belonging to the order Solanales by Cronquist (1981). Later, Thorne (1992) classified it as a member of the order Campanulales. In the APG IV system, it is classified as Asterales mostly due to its molecular-based characteristics (Angiosperm Phylogeny Group, 2016).
Approximately 28 families and 155 genera are known to exhibit floral dimorphism among angiosperms (Ganders, 1979). In general, if a flower element exhibits dimorphism, there are plants in which a form of pollen dimorphism also exists (Ganders, 1979). With regard to examples of floral dimorphism, Forsythia Vahl, Jasminum L., Abeliophyllum Nakai of Oleaceae, Damnacanthus indicus C. F. Gaertn (Rubiaceae), Pulmonaria L. (Boraginaceae), and Fagopyrum Mill. (Polygonaceae) have been reported (Hong and Han, 2002; Halbritter et al., 2018). Among heterostyly species, two different pollen types occur, where the pollen size and the number of apertures or the ornamentation may different. In Linum flavum L., the pollen shape of the thrum flower is baculate, whereas that of the pin flower is clavate (Halbritter et al., 2018). Also, in Primula veris L. (Primulaceae), the pollen of the thrum type is larger and has more apertures than the pollen of the pin type. The pin type has long styles, short filaments, small pollen grains, and large stigmatic papillae, whereas the thrum type has short styles, long filaments, large pollen grains, and small stigmatic papillae (Richards, 1986).
On the other hand, Armeria alpina Willd. (Plumbaginaceae) has pollen dimorphism, i.e., it is reticulate, with distinct sizes of the lumina and suprasculpture elements and is correlated with dimorphic stigmatic papillae, but the style and stamen lengths do not show dimorphism but area instead monomorphic (Ganders, 1979).
These are highly related to hydration in relation to the dimorphism of stigma papillae during pollen germination, which is considered to complete the incompatibility mechanism. In addition, heterostyly is a type of style polymorphism that has been described as the evolution of floral characters adapted for precision in terms of both pollen transfer and avoidance of pollen-stigma interference (Lloyd and Webb, 1992a, 1992b; Barrett, 2002b). There is a striking pattern of reproductive organ variation in several plant populations that are polymorphic in terms of the length or shape of the reproductive organs and the number of morphologically distinct mating groups within a population (Barrett, 2002b; Pauw, 2005).
In Menyanthaceae, heterostyly occurs in all genera except Liparophyllum. In addition, significant correlation between floral dimorphism and pollen dimorphism was confirmed statistically with Denmark and Greenland populations in Menyanthes trifoliata L. (Olesen, 1987). However, for other populations, the link between the occurrence of pollen dimorphism and floral dimorphism has not been studied. Therefore, this study aimed to investigate whether pollen dimorphism occurs when there is floral dimorphism in Korean populations.
MATERIALS AND METHODS
Pollen grains of Menyanthes trifoliata were collected from plants growing among wild populations in Korea. Three habitats are presented, as shown in Table 1. We refer to the population that served as the pollen source according to their habitats. M. trifoliata shows heterostyly in its reproductive system. Therefore, we collected pollen grains through separation into pin and thrum types. The pollen grains were placed in a 1.5 mL tube. For a scanning electron microscope (SEM) observation, the pollen grains were pre-fixed in 4% glutaraldehyde for 2 h and washed with 0.1 M phosphate buffer (pH 7.4). The fixed specimens were dehydrated using a 50, 70, 80, 90, 95, and 100% ethanol series. The dehydrated samples were substituted with a mixture of ethanol and isoamyl acetate at ratios of 2:1, 1:1, and 1:2 for 1 h. Then, the samples were substituted twice in 100% iso-amyl acetate every hour. The samples were treated with a hexamethyldisilazane (HMDS) solution for 1 h and dispensed on a glass slide with a pipette. After drying, the pollen grains were coated with platinum using an ion sputter system and observed by SEM (Hitachi, Tokyo, Japan). To obtain quantitative data pertaining to the pollen, 70 pollen grains of each style type were measured by a light microscope and analyzed statistically.
RESULTS
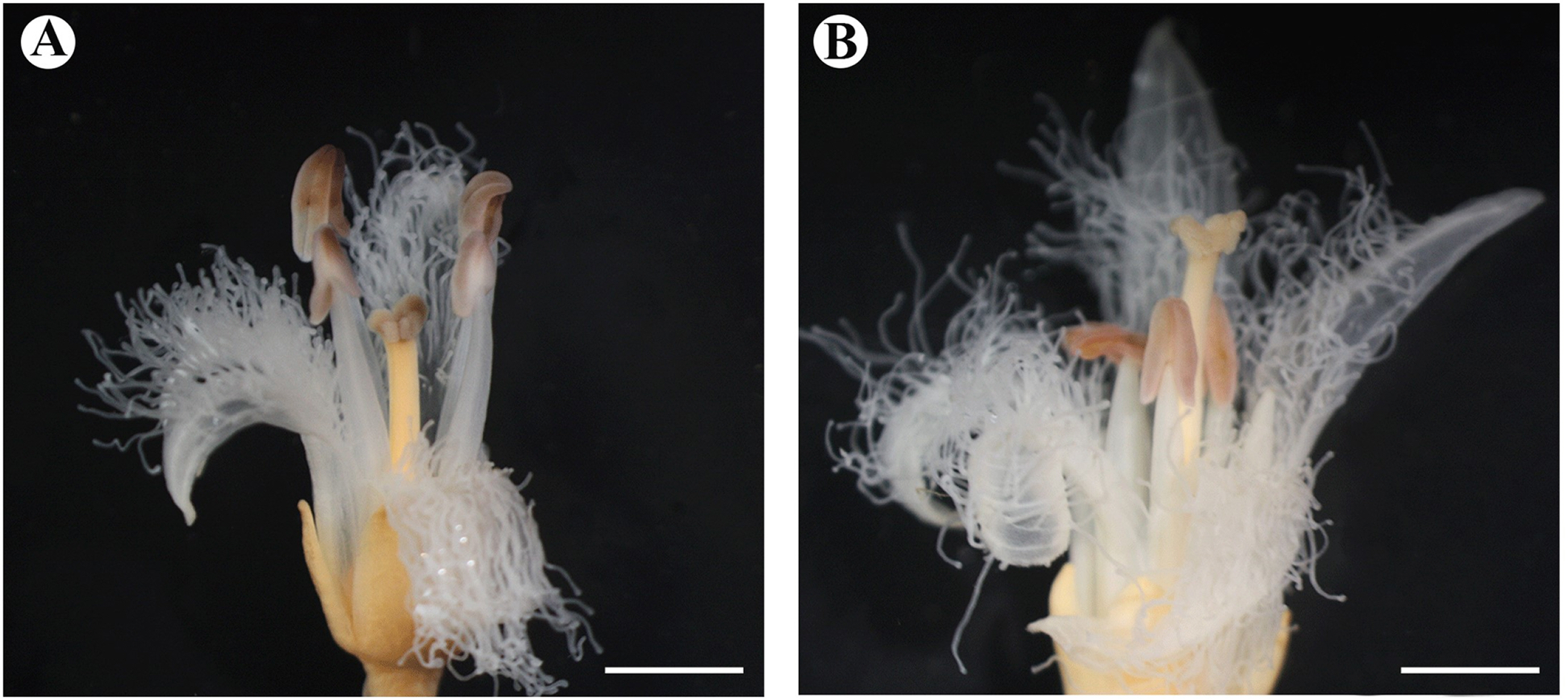
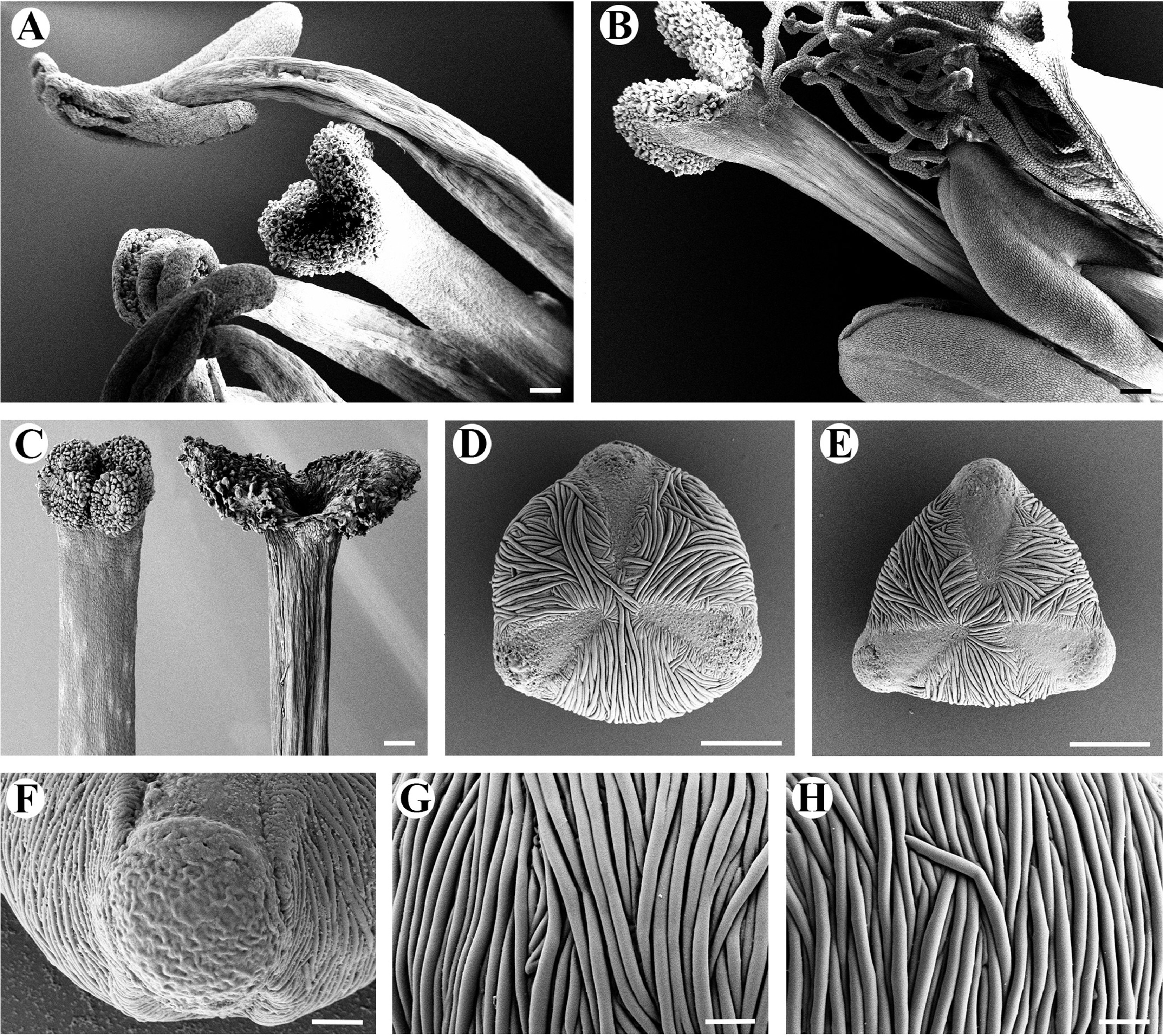
The floral morphology of Menyanthes trifoliata differed significantly in terms of the filament length, pistil length, and stigma shape (Fig. 1A, B). Homostyly was not found in the flowers. Stigmas in the pin and thrum flowers are bi-lobed with a papillate surface, with the papillae covering the abaxial surface (Figs. 1A, B, 2A–C). Both morphs have upright lobes and each lobe spread outwards. The size and shape of the stigma papilla are different between pin and thrum flowers (Fig. 1A, B). The style of the thrum flower is smaller than that of the pin flower (Figs. 1A, B, 2A–C). We also observed 70 pollen grains for M. trifoliata. Based on these observations, pin (long-style flower) and thrum (short-style flower) types of pollen grains appeared different in terms of the pollen size and shape (Fig. 2D, E, Tables 2, 3). Pollen grains of pin flowers showed means of 36.01 ± 2.01 μm, whereas those of thrum flowers showed means of 41.28 ± 2.58 μm in terms of the equatorial diameter (Fig. 2D, E, Table 3). Thus, the pollen grains of both flowers can be classified as medium-sized category (Table 2). The pollen dispersal unit was the monad type and the pollen polarity was isopolar. The pollen grains of both types were suboblate and tricolpate, with each furrow bearing a single germ pore (Fig. 2D–F, Table 2). From a polar view, the thrum type showed a convex-triangular shape and the pin type showed a triangular shape (Fig. 2D, E, Table 2). The exine sculpture is striate with a great number of dense, irregularly spaced ridges (Fig. 2G, H). The pollen grains of the two morphs have a relatively small apocolpial field at 5.62 ± 0.30 μm for the pin type and 6.24 ± 0.70 μm for the thrum type. The apocolpium index is 0.16 for the pin type and 0.15 for the thrum type (Fig. 2D, E, Table 3).
DISCUSSION
The reproductive floral morphology differed significantly in terms of the filament length, pistil length, and stigma shape. Menyanthaceae possesses style polymorphisms, mainly the distyly type, and members of the family have been studied to assess sexual system changes (Ornduff, 1966; Thompson et al., 1998; Tippery and Less, 2011a, 2011b). However, Nymphoides and Villarsia show not heterostyly but a slight homostyly condition. In relation to this, heterostyly had been found as an ancestral state (Tippery et al., 2008; Cohen, 2010). Heterostyly in M. trifoliata is understood as a mechanism to promote outcrossing as a survival strategy of the species (Barrett, 2002a, 2002b). Pin and thrum types can represent different functions of males and females (Barrett, 2002a, 2002b). Biased morphological ratios in heterostyly populations may be associated with the predominance of the reproductive mechanism (Barrett, 2019). In Menyanthaceae, homostyly and heterostyly populations are found in different habitats. Homostyly populations occupy a diversity of habitats ranging from large bodies of water to seasonal ponds and roadside depressions with a wider distribution, whereas heterostyly populations mostly appear in permanent water bodies (Haddadchi and Fatemi, 2015; Olesen, 1987). However, as opportunities for both types, cross-pollination via a pollinator ensures the reproduction and maintenance of the polymorphisms with equal morph frequencies within the populations (Haddadchi and Fatemi, 2015). We hypothesize that the heterostyly of M. trifoliata is associated with sexual reproduction and intra-morph incompatibility to preserve high genetic diversity has been found in Menyanthaceae. The shape and size of the stigma and papillae are highly relevant for pollen capturing and incompatibility issues, but the size and position are more frequently reported than the shape in heterostyly studies (Dulberger and Ornduff, 2000). Hence, a more stable environment selects for the persistence of the polymorphism. M. trifoliata have few habitats and are limited in terms of mating with other colonies. Given this situation, to preserve genetic diversity, the advantage of self-fertilization could be lost, whereas selection for reproductive assurance may favor mutation, thus increasing outcrossing compared to the pollen and flower morphological characteristics of the pin and thrum types in M. trifoliata.
According to Nic Lughadha and Parnell (1989), pollen sizes and shapes show differences in European populations found in Denmark and Greenland. They reported that the pollen size of the pin morph type is 40–45 × 20–25 μm, while that of the thrum type is 50–55 × 26–29 μm. The two morphs are both sub-prolate and tricolpate, with each furrow bearing a single germ pore. The ridges on thrum grains are more distinct than those on pin pollen grains. The furrows of pin-type grains appear to be more open than those of thrum-type grains (Nic Lughadha and Parnell, 1989). In the present study, however, the pollen characteristics differ from those in European populations. The Korean population has a suboblate shape and relatively small grains. Nevertheless, although M. trifoliata show differences in only the pollen size and shape when comparing European and Korean populations (Nic Lughadha and Parnell, 1989), heterostyly was also exhibited in the Korean M. trifoliata in terms of the pollen size and shape, stigma shape, and the apocolpium size. Therefore, like having distyly flowers, the pollen grains were also found to be a dimorphism in Korean populations of M. trifoliata.