동북아시아 뱀톱속 (석송과) 두 종의 분류학적 재검토
Taxonomic reexamination of two Huperzia species (Lycopodiaceae) in Northeast Asia
Article information
Abstract
Huperzia lucidula (Michaux) Trevis.의 동북아시아 집단 및 북미 집단을 대상으로 외부형태와 포자형태를 비교한 결과 잎의 형태, 엽연부, 잎에서의 기공의 분포 및 포자의 형태에 있어서 뚜렷히 구분되었다. 따라서, 동북아시아 집단을 북미 지역의 H. lucidula와는 독립된 종 H. asiatica (Ching) B.-Y. Sun and J. Lim으로 분리, 승격하였다. 또한, H. serrata (Thunb.) Trevis.와 동일 종 또는 종내 분류군으로 인식되어 오던 H. javanica (Sw.) C. Yang은 영양엽에서 엽병의 발달 유무, 엽연 거치의 수 그리고 무성아가지의 중앙 열편 모양으로 별개의 종으로 처리하는 견해를 지지하였다.
Trans Abstract
A comparison of the external morphology and spores clearly distinguished Huperzia lucidula (Michaux) Trevis. in northeast Asia and North America in terms of leaf shape, leaf margin, distribution of stomata on leaves, and spore shape. Therefore, the northeast Asian plants should be treated as a separate species, H. asiatica (Ching) B.-Y. Sun & J. Lim. In addition, we believe that H. javanica (Sw.) C. Yang, regarded as conspecific or infraspecific with H. serrata (Thunb.) Trevis., is a distinct species based on the presence of a leaf petiole and serrated leaf margins, and the shape of the gemmiferous branchlets.
Huperzia Bernh. (sensu stricto) includes about 55 taxa distributed in temperate, alpine, and arctic regions, and tropical Asian mountains (Zhang and Iwatsuki, 2013). There are four recognized taxa in Korea (Sun, 2007), four in northeast China (Zhang and Iwatsuki, 2013), eight in Japan (Iwatsuki et al., 1995), and five in Far East Russia (Kharkevich, 2002). The genus Huperzia is characterized by erection of stems, the presence of sporangia at the axils of fertile leaves, gemmiferous branchlets, and gemmae (Boivin, 1950; Wagner and Beitel, 1992, 1993; Sun, 2007; Zhang and Iwatsuki, 2013).
Lycopodium lucidulum [=Huperzia lucidula (Michaux) Trevis.] was described as a new species from North America based on spreading and narrowly lanceolate leaves with irregular teeth on the margin and sporangia at the axils of fertile leaves (Michaux, 1803). Ching (1981) described a new variety, H. lucidula var. asiatica Ching, based on a specimen collected on Mt. Jangbaek, Jilin Province, China, stating that its leaves were more reflexed, darker green, and smaller than those of H. lucidula. Recently, some authors have treated it as a synonym of H. lucidula, insisting that there are no obvious differences between the two taxa (Zhang and Kung, 2000; Zhang and Iwatsuki, 2013).
In 1801, Swartz published a new species, Lycopodium javanicum Sw. [= Huperzia javanica (Sw.) C. Yang], characterized by lanceolate or obovate leaves and dichotomously branched stems. However, Makino (1898) treated this taxon as a variety, L. serratum var. javanicum. Separately, Spring (1850) described L. serratum var. longipetiolatum Spring as a new variety, which was characterized by leaves forming petioles narrowing towards the base. Ching (1981) treated this taxon as a form of L. serratum Thunb., while Yang (1982) recognized it as a distinct species, H. longipetiolata (Spring) C. Yang, emphasizing that it has long petiolate leaves. Subsequently, he combined L. javanicum with H. longipetiolata, treating H. longipetiolata as a synonym of H. javanica (Yang, 1989), and Shrestha and Zhang (2015) concurred. However, Zhang and Kung (2000) and Zhang and Iwatsuki (2013) recognized this taxon as a variety of H. serrata. The distribution of L. serratum var. longipetiolatum in the Korean Peninsula has not been confirmed since Park (1961) first reported it.
In this study, we reexamined the taxonomic status of northeast Asian H. lucidula by comparing the morphology of northeast Asian and North American H. lucidula. In addition, we reexamined the taxonomic position of H. javanica, which has been recognized as conspecific with H. serrata. Finally, we verified the distribution of this species within the Korean Peninsula.
Materials and Methods
Plants were collected from various localities in Korea, China, Japan, and the USA from 2008 to 2014 (Table 1). Voucher specimens are kept in the Herbarium of the National Institute of Biological Resources (KB) and Chonbuk National University (JNU). In addition, we studied specimens on loan from the Chinese Academy of Sciences, Beijing (PE), New York Botanical Garden (NY), and Harvard University Herbaria (GH).
We examined the external morphology and used the results to compare species and describe each taxon in this study. We measured the number of stomata per 0.1 mm2 in the trophophores of 20 individuals from each taxon, as well as the size of 10 stomata per individual. We then calculated the minimum, average, and maximum number and size of the stomata. To examine spore micromorphology, we sampled spores from mature sporangia and immediately photographed them by scanning electron microscopy (SEM) without pretreatment.
Results and Discussion
Huperzia asiatica has been treated as conspecific or intraspecific with H. lucidula and is regarded as a good example of a disjunct distribution between northeast Asia and North America (Brunton et al., 1992; Zhang and Kung, 1998; Zhang and Iwatsuki, 2013; Xiang et al., 2015). However, on comparing the external morphology and spore micromorphology, these two taxa seem to be different enough to be treated as distinct species.
In terms of trophophyll morphology, the two species have different patterns. Huperzia lucidula typically has oblanceolate trophophylls with the above mid-part being the widest and irregular margins with 1−9 teeth, while H. asiatica always has linear-lanceolate trophophylls with the margins parallel from the base to the mid-part and 0−4 minute teeth or papillate margins restricted from the middle part to the end of the leaves (Fig. 1, Table 3). Since the delimitation of Huperzia species is based mainly on leaf shape and the serration of the leaf margin (Ching, 1981; Wagner and Beitel, 1993; Sun, 2007), even serration pattern has been used to distinguish sections Serratae and Huperzia (Zhang and Kung, 1998, 2000; Zhang and Iwatsuki, 2013). Consequently, the differences in trophophyll shape and marginal serration between these two taxa imply that they should be treated as different species.
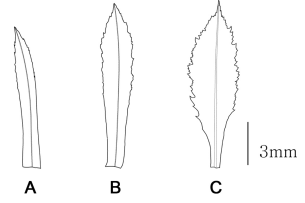
Shapes of trophophyll. A. Linear-lanceolate (H. asiatica); B. Oblanceolate (H. lucidula, H. serrata); C. Narrowly elliptic (H. javanica, H. serrata).
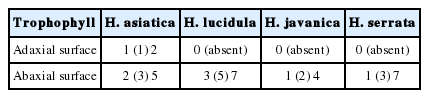
In addition, H. lucidula has stomata only on the abaxial leaf surface, whereas H. asiatica has stomata on both sides of the leaves (Fig. 4, Table 2). H. lucidula was described as a new species based on the characteristics of narrowly obovate leaves and stomata only on the abaxial surface of the leaf, and was regarded as distinct from other species in North America, which are characterized by linear-lanceolate leaves and stomata on both sides of the leaves (Wagner and Beitel, 1993). Lim and Sun (2015) suggested that the distribution of stomata on the leaf is a key character for identifying taxa within the genus Huperzia, reporting that H. serrata and H. javanica have stomata only on the abaxial leaf surface, while H. miyoshiana (Makino) Ching and H. jejuensis B.-Y. Sun & J. Lim have stomata on both sides of their leaves.
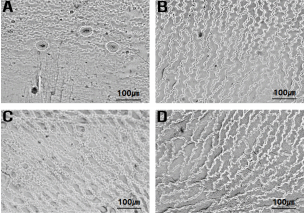
Light microscopic photographs of stomata at adaxial surface of leaf. A. H. asiatica; B. H. javanica; C. H. lucidula; D. H. serrata (Note that only H. asiatica has stomata on adaxial surface)
Regarding spore shape, H. lucidula has only normal triangular spores, while H. asiatica has a mixture of normal and irregular shapes which may indicate a hybrid origin (Fig. 5). Hybrids taxa are very common in this genus, and taxa with hybrid origins have a great variety of spore sizes and irregularly shaped spores (Knobloch, 1976; Wagner and Beitel, 1992, 1993).
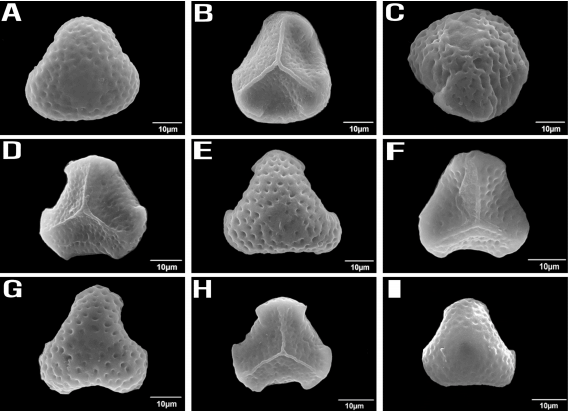
Scanning electron microscopic photographs of spore. A~C. H. asiatica; D~E. H. javanica; F~G. H. lucidula; H~I. H. serrata.
Since H. javanica was described as a new species based on its long leaf petioles, this taxon has been recognized as conspecific or intraspecific with H. serrata. Recently, Shrestha and Zhang (2015) treated it as distinct from H. serrata because of its petiolate trophophylls and reduced sporophylls. Our results also indicated that H. javanica is a separate species from H. serrata: H. serrata has narrowly elliptic or oblanceolate trophophylls with an acute or acuminate apex, 1−18 teeth on irregular margins, obovate central lobes (ratio of width to length > 0.2) of gemmiferous branchlets, and a mucronate apex of the gemmae leaf. In comparison, H. javanica has narrowly elliptic to elliptic trophophylls with a caudate apex, 10−25 teeth on an irregular margin, a linear central lobe (ratio of width to length < 0.2) on gemmiferous branchlets, and a cuspidate apex of the gemmae leaf (Table 3). In this study, we found that the central lobe shape of the gemmiferous branchlets is a useful character for distinguishing H. javanica from H. serrata. We also verified that this species occurs in the Sun-dol area of Jeju-do and Duryun-san at Haenam, based on an examination of specimens in the herbarium of the National Institute of Biological Resources (KB).
In conclusion, we recognize the northeast Asia populations, which Zhang and Kung (2000) and Zhang and Iwatsuki (2013) considered conspecific with H. lucidula, as a distinct species based on external morphology. Huperzia javanica should be treated as a distinct species independent of H. serrata. We also rediscovered it in the Korean Peninsula after it was first reported by Park (1961, 1979).
Taxonomic Treatment
1) Huperzia asiatica (Ching) B.-Y. Sun & J. Lim, stat. nov. (Fig. 2)

Huperzia asiatica (Ching) B.-Y. Sun & J. Lim. A. Habit, B. Fertile zone of stem, C. Trophophyll, D. Sporophyll, E. Gemma, F. Gemmiferous branchlet, G. Gemma attached at gemmiferous branchlet, H. Sporangia.
Huperzia lucidula var. asiatica Ching in Acta Bot. Yunnan. 3(3): 296. 1981. Type : China, Jilin, without date, S. L. Soong 197. (Holotype: PE)
H. lucidula (Michaux) Trevis. auct. non. Acta Phytotax. Sin. 38: 21. 2000.
Korean name: Baek-du-baem-top 백두뱀톱
Plant evergreen herb, terrestrial. Stems erect or ascending, subterranean stems decumbent, 1−2 dichotomously branched, 15.3−25.0 cm tall, together with leaves 1.3−1.8 cm wide, clustered at base without main stem; annual constrictions present. Gemmiferous branchlets present in 1 pseudowhorl at end of each annual growth cycle, zygomorphic, comprising 6 lobes, with 1 large central lobe (outer), 1 small central lobe (inner), and 2 pairs of small lateral lobes, large central lobe lanceolate, 2.6−4.0 × 0.5−0.8 mm; gemmae eliminated after maturity, obtriangular, 3.2−3.7 × 2.8−3.9 mm, zygomorphic, comprises 5 leaves, 1 central leaf, 2 large lateral leaves with apex cuspidate, 1 abaxial, and 1 adaxial leaf. Leaves monomorphic or dimorphic, attached at right angles or angled downward with stem, densely spirally arranged, green, herbaceous, glabrous; vein only midrib; stomata on both sides, 51.9−62.1 μm. Trophophylls linear-lanceolate, straight in lower half, 6.7−8.8 × 0.8−1.2 mm, apex acute, margins irregularly minutely dentate or papillate above middle, teeth 1−4. Sporophylls linear-lanceolate, 4.1−6.3 × 0.7−1.0 mm, slightly smaller than trophophylls, margins irregularly minutely dentate or papillate above middle, teeth 2−3, not forming distinct cones. Sporangia reniform, 0.6−1.1 × 1.2−1.6 mm, axillary of sporophylls, sessile, yellowish; spores trilete with truncate lobes, and irregular shape, 17−28 , foveolate, fossulate, scabrate.
Specimens examined: CHINA. Jilin: Mt. Baekdu, elev. 2040 m, 14 Jun. 2008, Doudkin s.n. (KB, 5 sheets); Mt. Baekdu, elev. 980 m, 10 Jun. 2014. Lee et al. baekdu 2307 (KB); Mt. Baekdu, elev. 892 m, 10 Jun. 2014. Lee et al. baekdu 2308 (KB); Mt. Baekdu, elev. 1780 m, 30 Jul. 1957, ? 466 (PE); Mt. Baekdu, elev. 1500m, 18 Jul. 1982, ? 4192 (2 sheets) (PE).
Distribution: Restricted to Mt. Beakdusan
Habitat: Terrestrial in shaded conifer forests and mixed forests, moist areas covered with many mosses.
2) H. javanica (Sw.) C. Yang, Bull. Acad. Mil. Med. Sci. 13: 368. 1989. (Fig. 3)

H. javanica (Sw.) C. Yang. A. Habit, B. Fertile zone of stem, C. Sporophyll, D. Trophophyll, E. Gemmiferous branchlets, F. Sporangia, G. Gemma.
L. javanicum Sw. J. Bot. (Schrader), 1800: 114. 1801; L. serratum var. javanicum (Sw.) Makino, Bot. Mag. Tokyo 12: 13. 1898. Type : Indonesia, Java, without date, Thunberg s.n. (Holotype: UPS)
L. serratum Thunb. var. longipetiolatum Spring, Monogr. Lycop. 2: 18. 1850; H. serrata Thunb. f. longipetiolata (Spring) Ching, Acta Bot. Yunnan 3: 294. 1981; H. longipetiolata (Spring) C. Yang, Chin. Trad. Herbal Drugs 16: 33. 1982.
Korean name: Kuen-baem-top 큰뱀톱
Plant evergreen herb, terrestrial. Stems erect or ascending, subterranean stems decumbent, 1−2 dichotomously branched, 9.2−23.8 cm tall, together with leaves 2.0−3.9 cm wide, clustered at base without main stem; annual constrictions present. Gemmiferous branchlets present in 1 pseudowhorl at end of each annual growth cycle, zygomorphic, comprising 6 lobes, with 1 large central lobe (outer), 1 small central lobe (inner), and 2 pairs of small lateral lobes, large central lobe lanceolate, 2.7−4.3 × 0.5−0.7 mm; gemmae eliminated after maturity, obtriangular, 2.4−3.5 × 2.3-3.6 mm, zygomorphic, comprises 5 leaves, 1 central leaf, 2 large lateral leaves with apex cuspidate, 1 abaxial, and 1 adaxial leaf. Leaves dimorphic, attached at right angles or angled downward with stem, densely spirally arranged, dark green, herbaceous, glabrous; vein only midrib; stomata on lower side only, 51.4− 70.9 μm. Trophophylls narrowly elliptic or elliptic, broadest at mid-part, 10.8−18.8 × 1.9−4.2 mm, apex caudate, margins irregularly serrate, teeth 10−25. Sporophylls linear-lanceolate or lanceolate or obovate, variable in form, 2.1−5.0 × 0.3− 1.0 mm, margins irregularly serrate, in whole part, teeth 21- 10, not forming distinct cones. Sporangia reniform, 0.6− 1.1 × 1.2−1.6 mm, axillary of sporophylls, sessile, yellowish; spores trilete with truncate lobes, 13−22 μm, foveolate, fossulate, scabrate.
Specimens examined: CHINA. Anhui: Mt. Hwang, Songkokam, 2 Nov. 1951, ? 6501 (PE); Fujian: Jianglexian, elev. 550m, 22 May 1991, ? 279 (PE); Guizhou: Anlongxian, Mt. Long, 15 Jun. 1960, Zhang & Zhang 4529 (PE); Hunan: Sangzhixian, Mt. Cheonpyeong, elev. 1370, Oct. 1987, Moon 870514 (PE, 2sheets); Wugangsi, Mt. Wun, elev. 1000m, 13 Oct. 1963, ? 15957 (PE); Yizhangxian, Mt. Mang, elev. 900m, Feb. 2005, ? 602017 (PE, 2 sheets); Chalingxian, elev. 670m, 2 Dec. 2007, ? 70455 (PE); Jiangxi: Hwangkang, elev. 750m, 25 Aug. 1959, ? 1633 (PE); Sichuan: Muchuanxian, Mt. Wuzhi, elev. 1100m, 7 May 1976, ? s.n. (PE); Zhejiang: Longquan, Mt. Pyeongan, elev. 1100m, 18 Aug. 1983, ? 3253 (PE).
JAPAN. Honshu: Shoshazan, Harima, elev. 200m, 10 Nov. 1975, Y. Saiki s.n. (PE); Nakasaki: Tsushima, 4 May 2014, Moon 1.(KB).
KOREA. Jeju-do: Seogwipo-si, Sanghyo-dong, Seondol, 18 Sep 2011, Hwang et al. 60580, 60582 (KB); Seogwipo-si, Sanghyo-dong, Seondol, 15 Dec. 2008, Kim J. O. EWLEE080564 (KB); Seogwipo-si, Sanghyo-dong, Seondol, 19 Sep. 2009, Lee S. J. 09-P312 (KB); Jeollanamdo: Jangheung-gun, Mt. Duryun, 23 Apr 2010, Hwang & Choi CH50153 (KB).
Distribution: Korea, Laos, Malaysia, Vietnam, India, Japan, China, Taiwan, Philippines (Iwatsuki et al., 1995; Zhang and Iwatsuki, 2013; Shrestha and Zhang, 2015)
Habitat: Terrestrial in shaded conifer forests and mixed forests, moist areas covered with many mosses.
Acknowledgements
This research was supported by a grant from the National Institute of Biological Resources (NIBR), the Ministry of Environment (MOE) Republic of Korea (NIBR201501202, NIBR201502201).