Taxonomic implications of floral morphology in the subfamily Asclepiadoideae (Apocynaceae s.l.) in Korea
Article information
Abstract
We examined the floral morphology of 15 taxa of five genera (Cynanchum, Marsdenia, Metaplexis, Tylophora, and Vincetoxicum) in Korean Asclepiadoideae using a stereoscopic and scanning electronic microscope to clarify and describe the floral characteristics. In this study, the corolla and corona types, the types of corona lobes, appendages on the corona lobes, and the apex shape of the style head are considered as diagnostic characteristics at the generic level. The genus Vincetoxicum, which is treated as a synonym of the genus Cynanchum in Korea, is distinguished from Cynanchum by the interstaminal part on the corona, fleshy and mainly triangular or ellipsoid corona lobes, and various corolla colors. In Cynanchum, various corona types have been observed, while Vincetoxicum have similar corona types among the taxa. In addition, the main floral characteristics at the species level were as follows: flowering time, inflorescence, corolla color, trichomes on the corolla surfaces, apex shape of the corona lobe, and trichomes on the ovary. This study presents the taxonomic importance of floral morphology by providing descriptions and diagnostic characteristics among the genera and species investigated.
Asclepiadoideae, the largest subfamily in the family Apocynaceae, is composed of approximately 190 genera and more than 2,500 species distributed from tropical to temperate regions and characterized by the possession of pollinia (Endress and Stevens, 2001). According to the most recent classification, Apocynaceae s.l. is recognized by the pollen type in the five subfamilies of Rauvolfioideae, Apocynoideae, Periplocoideae, Secamonoideae, and Asclepiadoideae (Endress and Bruyns, 2000). Moreover, many phylogenetic studies support the contention that traditional Asclepiadaceae is not a monophyletic group and thus should be treated as a subfamily, i.e., Asclepiadoideae within Apocynaceae s.l. (Sennblad and Bremer, 1996, 2002; Liede, 1997; Civeyrel et al., 1998; Endress and Stevens, 2001; Potgieter and Albert, 2001; Endress, 2004; Goyder et al., 2007; Rapini et al., 2007).
Members of Asclepiadoideae have specialized floral structures such as pollinia, gynostegia, and corona. Pollinia are an aggregate of many integrated pollen grains that are removed as a whole from anthers during the pollination process (Johnson and Edwards, 2000; Harder and Johnson, 2008). Each pollen grain of pollinia has clear implications with regard to plant reproduction; thus, having pollen masses improves the reproductive performance (Harder and Johnson, 2008). The gynostegium is formed by the post-genital fusion of the androecium and gynoecium, and pollinia are deposited onto the apex of the swollen head, called a style head (Fishbein, 2001). The corona has various types and shapes arising from corolla (corolline corona) or androecium (staminal corona). Corolline corona can be found in Rauvolfioideae, Apocynoideae, and Periplocoideae, whereas most Asclepiadoideae and Secamonoideae exhibit staminal coronas. Corolline coronas are also present in basal taxa such as Fockeeae and Marsdenieae (Liede and Kunze, 1993; Kunze, 2005).
The types of pollinia and corona are generally considered to be the most important characteristics for the classification of Asclepiadoideae (Liede and Kunze, 1993). Within the subfamily Asclepiadoideae, four tribes (Asclepiadeae, Gonolobeae, Marsdenieae, and Ceropegieae) are usually recognized according to the orientation of the pollinia, appendages on the margins of the pollinia, and the original position of the corona (Bruyns and Forster, 1991; Endress and Bruyns, 2000). Following Schumann’s hypothesis on subtribal circumscription using corona characteristics only (1895), Liede (1997) classified the subtribes into six types (Asclepiadinae, Astephaninae, Glossonematinae, Gonolobinae, Metastelmatinae, and Oxypetalinae) based on the life form, root type, gynostegium, and corona type.
Many authors have investigated morphological characters of the complicated flowers in Asclepiadoideae. Frye (1902) examined the floral development of Asclepias L. in South America and reviewed mistaken characteristics and functions in detail. Liede and Kunze (1993) provided a basic system of various corona types and described four corona types and their diverse combinations. Endress and Bruyns (2000) illustrated the main floral characteristics of five subfamilies in Apocynaceae s.l. using scanning electron microscopy (SEM), and presented a comprehensive classification for subfamilies and tribes. More recently, Wiemer et al. (2012) investigated the functional floral morphology of South American Asclepias correlated with pollination biology. Although many studies have examined the floral characters of Asclepiadoideae, in general they failed to include a comprehensive taxon sampling. In particular, Asian species were scarcely considered in any of these studies.
In South Korea, 16 taxa in five genera (Cynanchum L., Metaplexis R. Br., Tylophora R. Br., Vincetoxicum Wolf, and Marsdenia R. Br.) have been generally described (Lee, 1996; Lee, 2003; Chang et al., 2014). Among the taxa, three (Vincetoxicum amplexicaule Siebold & Zucc., V. japonica (C. Morren & Decne.) Decne., and Marsdenia tomentosa C. Morren & Decne.) and two (V. inamoenum Maxim. and Tylophora floribunda Miq.) species are categorized as endangered and vulnerable, respectively, in the Rare Plants Data Book in Korea (Korea National Arboretum, 2008). Among the genera, Cynanchum and its related genus Vincetoxicum have long been debated with reference to their taxonomic position, but many recent studies have asserted that Vincetoxicum is an independent genus based on chemotaxonomic, morphological, and phylogenetic studies (Ali and Khartoon, 1982; Qiu et al., 1989; Forster, 1991; Liede, 1996, 1999; Potgieter and Albert, 2001; Khanum et al., 2016).
To date, a comprehensive taxonomic study of Korean Asclepiadoideae is lacking; thus, the complex floral structure remains unclear and its description is lacking. The objectives of this study are to describe the complicated floral structures, identify diagnostic floral characters, and elucidate taxonomic relationships among the genera and species in Korean Asclepiadoideae.
Materials and Methods
To examine the floral morphological characters, fifteen taxa in five genera (Cynanchum, Metaplexis, Tylophora, Vincetoxicum, and Marsdenia) were collected in Korea from April of 2014 to September of 2016, and the voucher specimens were deposited in the herbarium of Andong National University (ANH) (Table 1). Observations and measurements of the floral characteristics were conducted using fixed samples in 70% ethanol, and more than twenty flowers of each taxon were measured and photographed using a stereoscopic microscope (Olympus AX-70, Tokyo, Japan; Olympus DP2-BSW, Hamburg, Germany). Floral terms were adopted from various studies (Frye, 1902; Woodson, 1941; Liede and Kunze 1993; Endress and Bruyns, 2000) and the corona terms followed Liede and Kunze (1993) and Kunze (2005). The floral parts are illustrated in Fig. 1.
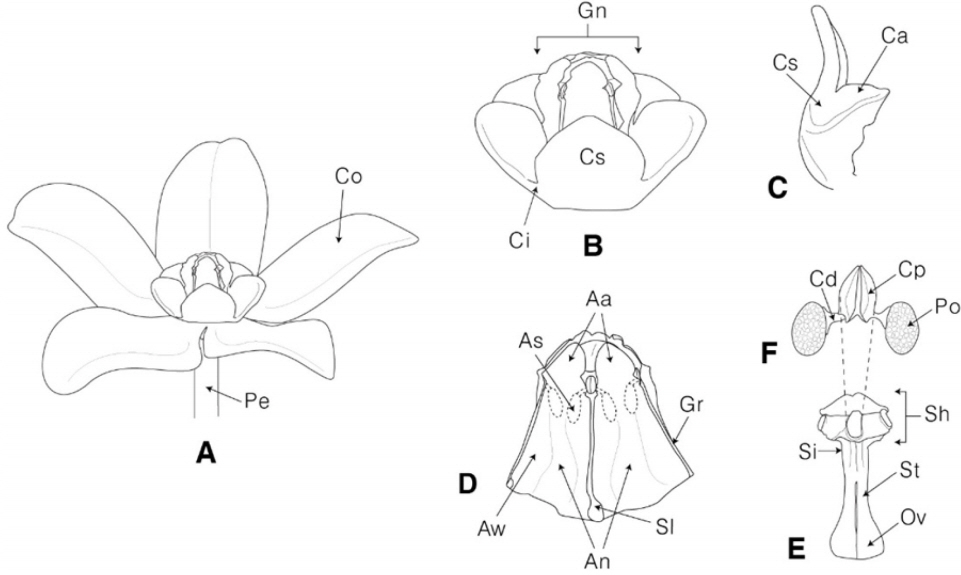
Illustrations of floral parts in Korean Asclepiadoideae. A. Flower. B. Gynostegium with corona. C. Staminal corona with appendage. D. Gynostegium. E. Gynoecium. F. Pollinarium. Aa, anther appendage; An, anther; As, anther sac; Aw, anther wing; Ca, appendage on corona lobe; Cd, caudicle; Ci, interstaminal part of corona; Co, Corolla; Cs, staminal corona lobe; Cp, corpusculum; Gn, Gynostegium; Gr, Guide rail; Ov, ovary; Pe, pedicel; Po, pollinium; Sh, style head; Si, stigma; Sl, slit; St, style.
For the SEM, flower samples were fixed in 1.5% glutaraldehyde for two hours. Fixed samples were washed twice with a phosphate buffer solution (pH 6.8) and were gradually dehydrated in an ethanol series (25 to 100%), after which they were stored in isoamyl acetate. Samples were critical-point dried (HCP-2), mounted on stubs, coated with gold by means of ion sputtering (thickness: 200–250 Å), and observed with a SEM (JSM-6300, Jeol, Tokyo, Japan; 15Kv; working distance; 35 mm).
Results
Flowering time
The flowering times allowed the creation of three distinct groups. First, four species bloom between May and June (Vincetoxicum acuminatifolium (Hemsl.) B. M. Nam & G. Y. Chung, V. atratum (Bunge) C. Morren & Decne. ex Decne., V. inamoenum, and V. japonicum), and five species flower from July to August (Cynanchum wilfordii (Maxim.) Hemsl., Tylophora floribunda, Vincetoxicum amplexicaule, V. nipponicum (Matsum.) Kitag., and V. pycnostelma Kitag.). Lastly, from August to September, reproductive characters in the remaining taxa (Cynanchum boudieri H. Lév. & Vaniot, C. chinense R. Br., Metaplexis japonica (Thunb.) Makino, Vincetoxicum glabrum (Nakai) Kitag., V. volubile Maxim., and Marsdenia tomentosa) can be observed.
Inflorescence
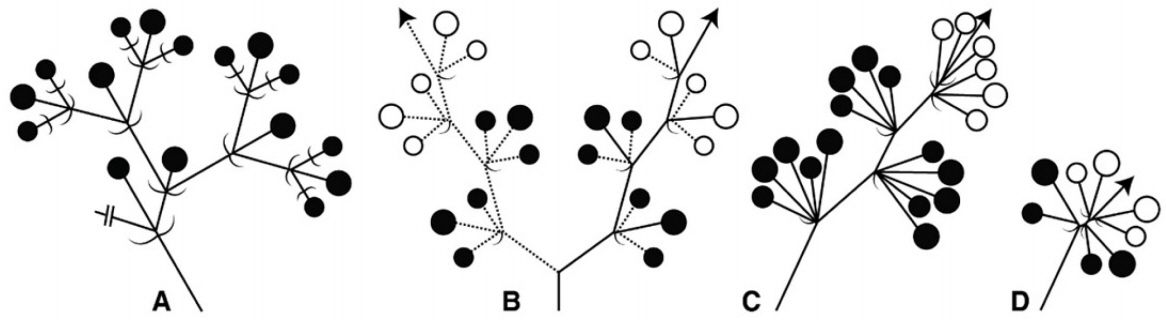
Inflorescences of the studied taxa are developed mainly on axillary and/or terminal parts. The flower numbers per inflorescence range from 3 to 29 on average (Table 2). C. wilfordii shows the highest number of flowers, whereas V. nipponicum has the fewest flowers. The peduncle lengths range from 0.7 to 62.1mm (Table 2). The inflorescence type is basically a cyme (Fig. 2) which can be divided into four types: dichasial (T. floribunda, V. acuminatifolium, V. pycnostelma) (Fig. 2A), scorpioid (C. chinense) (Fig. 2B), thyrse (C. boudieri, M. japonica) (Fig. 2C), and umbellate (C. wilfordii, V. amplexicaule, V. atratum, V. glabrum, V. inamoenum, V. japonicum, V. nipponicum, V. volubile, and M. tomentosa) (Fig. 2D).
Corolla
Two corolla types, rotate and tubular, are observed in this study, and all taxa except Marsdenia tomentosa exhibit a rotate corolla (Fig. 3).
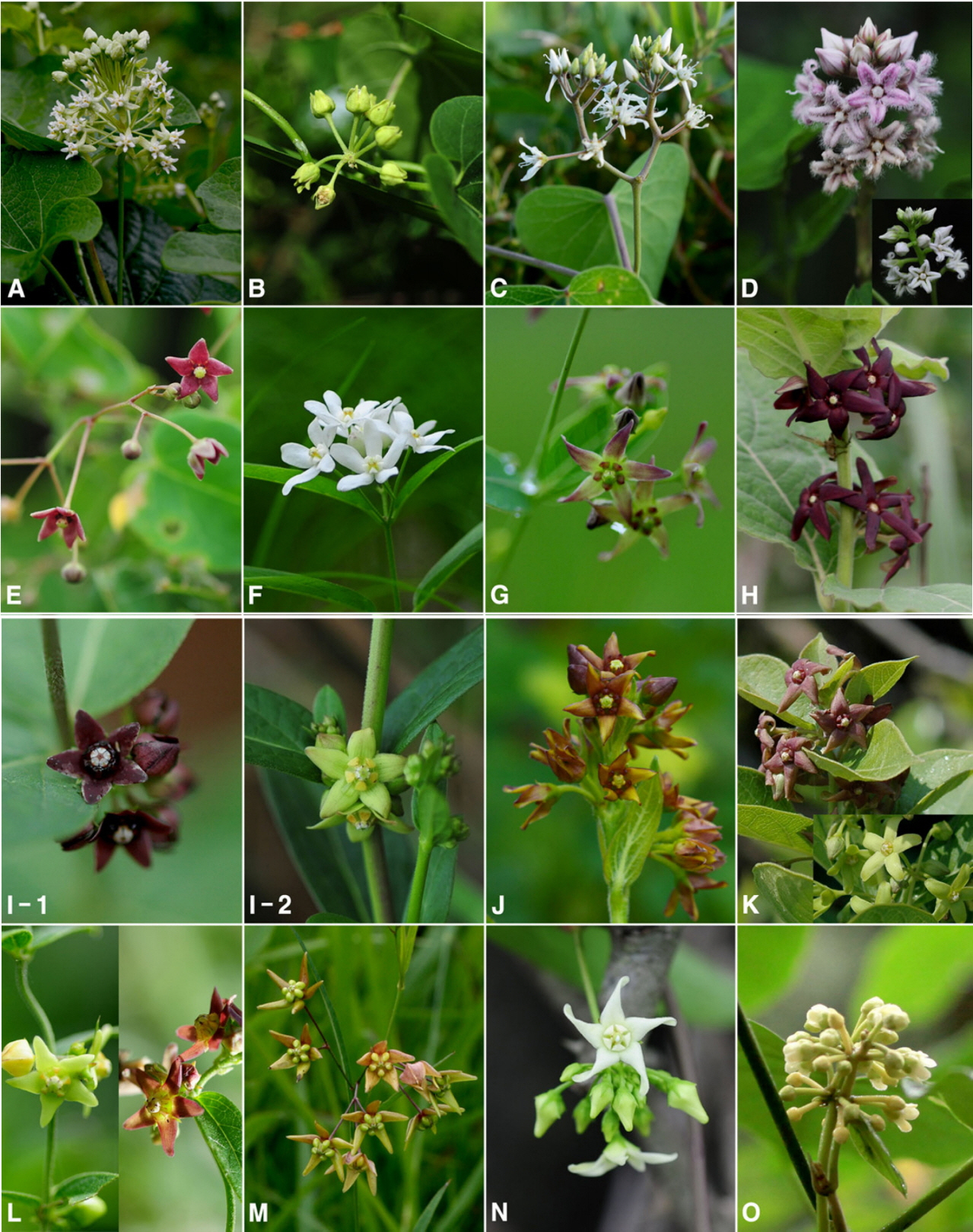
Variation of corolla colors in Korean Asclepiadoideae. A. Cynanchum boudieri. B. C. chinense. C. C. wilfordii. D. Metaplexis japonica. E. Tylophora floribunda. F. Vincetoxicum acuminatifolium. G. V. amplexicaule. H. V. atratum. I. V. glabrum. J. V. inamoenum. K. V. japonicum. L. V. nipponicum. M. V. pycnostelma. N. V. volubile. O. Marsdenia tomentosa.
Five shapes of corolla lobes are recognized: narrowly lanceolate, triangular, triangular-ovate, oblong, and semi-circular (Table 2). In the case of C. boudieri, the corolla lobes were strongly reflexed (Fig. 3A), and most taxa in Vincetoxicum show corollas which are twisted in a clockwise direction. In addition, corolla lobes with curled tips are found only in M. japonica (Fig. 3D).
Corolla colors are white, pale pink, yellowish, yellowish green, and purplish brown (Table 2), but some taxa have variation within individuals or populations (Fig. 3). Most taxa of Vincetoxicum and Tylophora have two different colors: yellowish and purplish brown, except for V. acuminatifolium and V. volubile, which are white (Fig. 3E–N), although sometimes gradational color changes occur in the same individuals (Fig. 3G, J, M). In the Cynanchum, Marsdenia, and Metaplexis taxa, white, pale pink and yellowish green colors are observed (Fig. 3A–D, O).
Trichomes on the adaxial surface of the corolla are found in C. boudieri, C. wilfordii, M. japonica, T. floribunda, V. amplexicaule, V. volubile, and M. tomentosa (Table 2). The positions of the trichomes vary among the taxa. Metaplexis japonica is covered by densely flexuous trichomes on the whole of the corolla lobes, and T. floribunda and V. amplexicaule are covered by short trichomes on the basal parts of the corolla lobes. Trichomes on the abaxial surface of the corolla are found only in V. atratum. However, Marsdenia tomentosa has short wooly trichomes inside the throat of the corolla.
Corona
In Korean Asclepiadoideae, corona types are largely divided into three types: free corona, corona with fused staminal and interstaminal parts, and ring-like corona (Table 2). Free coronas consisting of only staminal corona lobes (usually five free lobes) are observed in T. floribunda, V. pycnostelma, and M. tomentosa (Fig. 4E, M, O). In eleven taxa of Cynanchum and Vincetoxicum (Table 2), the interstaminal part is present and from the basal region almost to the gynostegium fused with the staminal corona lobes (Fig. 4). In Cynanchum, the interstaminal part is deeply lobed in C. boudieri and C. wilfordii (Fig. 4A, C). On the other hand, the interstaminal part of C. chinense is similar to the gynostegium in height (Fig. 4B). The margin of the interstaminal part of C. boudieri is revolute (Fig. 4A). In Vincetoxicum, most of the investigated taxa (except V. pycnostelma) are deeply lobed (Fig. 4F–N), but V. glabrum and V. volubile are observed to be lobed at about half of the height of the gynostegium (Fig. 4I, N). Lastly, a ring-like corona of M. japonica is developed along the basal part of the gynostegium, and it is very short and vestigial (Fig. 4D).

Shapes of corona and gynostegia using scanning electron microscopy in Korean Asclepiadoideae. A. Cynanchum boudieri. B. C. chinense. C. C. wilfordii. D. Metaplexis japonica. E. Tylophora floribunda. F. Vincetoxicum acuminatifolium. G. V. amplexicaule. H. V. atratum. I. V. glabrum. J. V. inamoenum. K. V. japonicum. L. V. nipponicum. M. V. pycnostelma. N. V. volubile. O. Marsdenia tomentosa. Arrow indicates stipe.
Staminal parts were usually longer and thicker than interstaminal parts. Two types of staminal corona lobes are found: laminar and fleshy (Table 2). The laminar types were also observed in Cynanchum (Fig. 4A–C). Among them, C. boudieri and C. chinense have staminal corona lobes with fleshy ligulate appendages and flattened long and slender appendages adaxially, respectively. The fleshy types are found in Tylophora, Vincetoxicum, and Marsdenia (Fig. 4E–O). They usually have triangular and apex shapes that are rounded and acute. The sizes of the staminal corona lobes vary (Table 2), and this is a useful characteristic by which to identify taxa at the interspecies level.
Gynostegium
The gynostegium is sessile or stipellate, and most of the taxa studied here showed sessile gynostegia. A gynostegium atop a stipe is observed in C. wilfordii, T. floribunda, and M. tomentosa, with their coronas fused to the stipe (Fig. 4C, E, O).
The style head, the apex part of the gynostegium, is covered by five membranous anther appendages. Two shapes of these anther appendages are recognized as ovate (to widely ovate) and transversely elliptic (Table 2). The apex shapes of the style head are divided into four types: flat, umbonate, conical, and elongated (Fig. 5, Table 2). Only M. japonica is characterized as having an elongated style head twisted apically. In three taxa examined, C. chinense, C. wilfordii, and M. tomentosa, all have two papillae on the apex of the style head (Fig. 5B, C, O).

Shapes of gynoecia and style heads in Korean Asclepiadoideae. A. Cynanchum boudieri. B. C. chinense. C. C. wilfordii. D. Metaplexis japonica. E. Tylophora floribunda. F. Vincetoxicum acuminatifolium. G. V. amplexicaule. H. V. atratum. I. V. glabrum. J. V. inamoenum. K. V. japonicum. L. V. nipponicum. M. V. pycnostelma. N. V. volubile. O. Marsdenia tomentosa. Arrow indicates papillae.
Trichomes on the surface of the ovary are found only in Vincetoxicum atratum (Fig. 5H).
Discussion
There is no doubt that floral characteristics play an important role when classifying Asclepiadoideae (Liede and Kunze, 1993; Liede and Weberling, 1995; Kunze, 1996; Endress and Bruyns, 2000; Fishbein, 2001; Ollerton and Liede, 2003; Kunze, 2005). The results of the present investigation, which includes 15 taxa in five genera of Asclepiadoideae from Korea, show that the diverse floral characteristics in Asclepiadoideae were useful to determine the delimitation of both genera and species. At the generic level, corolla and corona types, the type of corona lobe, appendages on the corona lobe, and the apex shape of the style head were considered as key characteristics for the Korean taxa (Table 3). Two genera, Cynanchum and Vincetoxicum, are known to have similar external and pollen morphology types (Liede, 1999, Nam and Chung, 2015). However, in this study, Cynanchum was found to have various shapes of the corona lobe with/without an appendage adaxially, as well as a laminar type of the corona lobes, whereas Vincetoxicum is characterized as having mainly fleshy triangular corona lobes without appendages and various corolla colors (Table 3). To clarify the circumscription between genera, morphological research on the vegetative organs and phylogenetic studies are required.
The corolla color provided useful information for identifying species immediately, but color changes occurred within individuals or populations (Fig. 3). In Korean Asclepiadoideae, the corolla color has been used to identify several infra-species types, such as V. amplexicaule var. castaneum (Makino) Kitag. and C. nipponicum var. glabrum (Nakai) H. Hara, which are described only in terms of the corolla color. However, in the present study, continuous variation was observed even within the same individual, thus further study on corolla color is needed. A tubular corolla was observed only in M. tomentosa, while the remaining taxa had a rotate corolla. Among the Korean Asclepiadoideae, the corolla type provides a diagnostic characteristic for Marsdenia; however, campanulate and rotate corollas are also present in the genus (Meve et al., 2017). Trichomes on the adaxial surface of the corolla have been recognized as one of the synapomorphic characteristics in the subtribe Astephaninae (among 19 genera, only Vincetoxicum and Tylophora are included) (Liede, 1994). However, trichomes on the corolla are observed only in two species of Vincetoxicum (V. amplexicaule and V. volubile) among the nine taxa and Tylophora. Thus, this characteristic should be reconsidered in order to understand the evolution of the subtribe.
Despite the fact that corona features have frequently been used for taxonomic classification in the subfamily, it is difficult to utilize these features in all of the taxa. One of the reasons is that corona types and their combinations are highly diverse and complicated. Moreover, the terms of these complex corona shapes can differ depending on the author. According to Liede and Kunze (1993), who have suggested various corona types and their evolution in Asclepiadaceae (=Asclepiadoideae), four basic corona types (corolline corona, staminal corona, interstaminal corona, and annular corona) and their combinations were described. Among the types, staminal coronas and combinations of staminal and interstaminal coronas are observed in the Korean Asclepiadoideae (Fig. 4, Table 2). In most taxa of Cynanchum and Vincetoxicum (except V. pycnostelma), a combination of a corona consisting of staminal lobes with a connected interstaminal part was observed (Fig. 4A–C, F–N). However, V. pycnostelma, Tylophora and Marsdenia had only staminal corona lobes (Fig. 4E, M, O). In addition, Metaplexis japonica had a vestigial corona (Fig. 4D). Khanum et al. (2016) noted that Cynanchum included various types of coronas; free corona and reduced corona types are also developed within the genus. Thus, classification only by means of the corona characteristics is inadequate. Therefore, a reexamination covering all taxa should be conducted to clarify precise, applicable morphological terminology for the unique taxa and their classification.
The function and homology of the coronas in Asclepiadoideae are little known. The role of the corona may be to lead pollinators into the guide rail (Ollerton and Liede, 2003) and/or to store nectar on the interstaminal part of the corona (Monteiro and Demarco, 2017). According to Ollerton and Liede (2003), if the corona is reduced or missing, its role can be filled by the trichomes inside the corolla. Most taxa in Cynanchum and Vincetoxicum have a conspicuous interstaminal part on the coronas, and nectar was detected during fieldwork (in fresh materials). On the other hand, Metaplexis japonica has a vestigial corona which is very short and ring-shaped (Fig. 4D), and a reduced corona was present in Marsdenia tomentosa (Fig. 4O). These species have conspicuous trichomes on the corolla (Table 2). These observations are congruent with the hypothesis of Ollerton and Liede (2003). Further investigations of the interaction between coronas and pollinators are necessary for a better understanding of their floral functions and pollination roles. The unique floral morphology in the subfamily provides an opportunity to understand the coevolution of flowers and insects, as well as the currently thriving biodiversity.
A style head which is fused with anthers forming a gynostegium has been considered as a non-fertile part, and the stigma is placed under part of the style head (Endress, 2016). The shape of the style head is considered to be an important characteristic in some taxa. Metaplexis, distributed in East Asia with only two species, is characterized by a ring-like corona and an elongated style head (Khanum et al., 2016). However, an elongated style head occurs in either Cynanchum or Marsdenia (Khanum et al., 2016; Meve et al., 2017), and Metaplexis hemsleyana Oliv. (distributed in China) exhibits a conical style head (Li et al., 1995). Although several studies of the pollination of Metaplexis japonica have been conducted (Sugiura and Yamazaki, 2005; Tanaka et al., 2006), their floral structures, especially their corona shapes, remain unclear. Thus, the floral structure of Metaplexis, including Chinese taxa, also requires further studies.
Considering the diverse floral characteristics described in relation to the members of Asclepiadoideae in Korea, the corolla and corona type, the type of corona lobe, appendages on the corona lobe, and the apex shape of the style head are useful for generic classification. Vincetoxicum (except V. pycnostelma) is morphologically distinctive with a fleshy staminal corona lobe which is interstaminally connate. In general, morphological studies of Asclepiadoideae have relied on work mainly done in Europe, Africa, and North America, and studies of generic and specific relationships within Asclepiadoideae are still insufficient. Therefore, a comprehensive investigation including morphological and molecular characteristics should be conducted to clarify their classification and to elucidate the phylogenetic relationships among the genera and species.
Acknowledgements
Authors thank the anonymous reviewers for their valuable comments and suggestions. This study was supported by a research grant of Andong National University (2017).
Notes
Conflict of Interest
Authors declare that there are no conflicts of interest.