Allium ulleungense (Amaryllidaceae), a new species endemic to Ulleungdo Island, Korea
Article information
Abstract
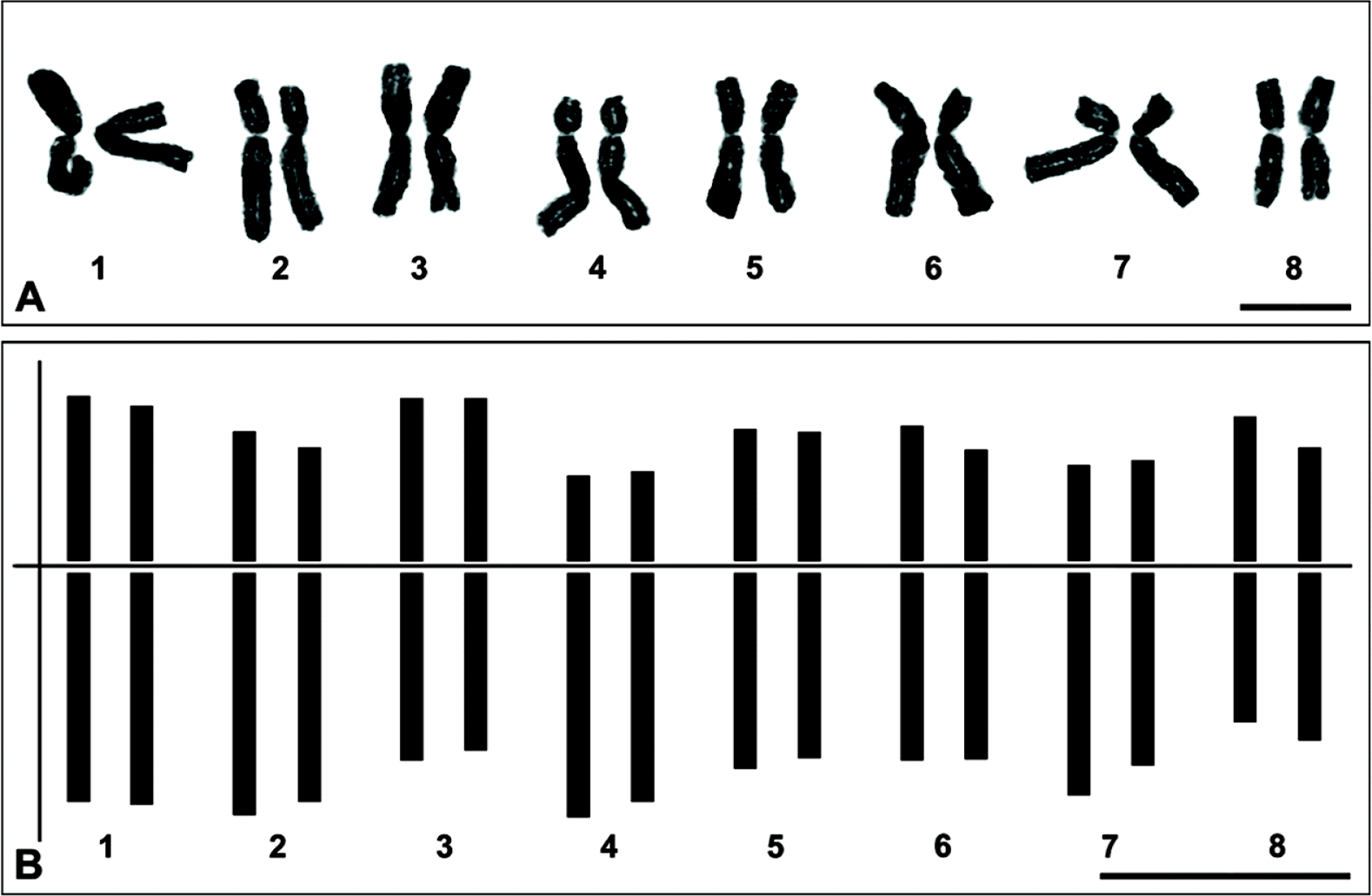
Allium ulleungense (subg. Anguinum, Amaryllidaceae), from Ulleungdo Island, Korea, is described as a new species. It is clearly distinguished from its close relatives, A. microdictyon and A. ochotense, by its broader leaves and larger whitish perianth and by its diploid chromosome number, which is 2n = 2x = 16. The lengths of the chromosomes range from 11.3 to 15.75 μm. Molecular phylogenetic analyses using nuclear and chloroplast markers also clearly indicate that A. ulleungense is genetically distinct from other species of the subg. Anguinum.
With over 900 species (Seregin et al., 2015), Allium L. is one of the largest genera in the Amaryllidaceae (Friesen et al., 2006; Fritsch et al., 2010; Li et al., 2010). It is characterized by bulbs enclosed in membranous (sometimes becoming fibrous) tunics, free or almost free tepals, and often a subgynobasic style (Friesen et al., 2006). Most taxa produce remarkable amounts of cysteine sulphoxides causing the well-known characteristic odor and taste (Friesen et al., 2006). Allium is distributed naturally in the northern hemisphere and in South Africa, mostly in regions with dry seasons (de Sarker et al., 1997; Friesen et al., 2006; Nguyen et al., 2008; Neshati and Fritsch, 2009). The classification of Allium by Friesen et al. (2006) based on molecular phylogenetic analyses includes 15 subgenera and 56 sections. About 19 taxa, excluding cultivated species, are known from the Korean peninsula (Choi and Oh, 2011; Shukherdorj et al., 2018).
Allium subg. Anguinum (G. Don. ex W. D. J. Koch) N. Friesen consists of ten species and several varieties and is part of the second of three main evolutionary lines of Allium (Fritsch, 2001; Fritsch and Friesen, 2002; Friesen et al., 2006; Li et al., 2010; Choi et al., 2012). The distribution area of subg. Anguinum reaches from southwestern Europe to eastern Asia and northeastern North America (Herden et al., 2016). Except for A. victorialis L. in Europe and A. tricoccum Solander in North America, all the other species of subg. Anguinum are in Asia (Herden et al., 2016). The habitats of the species of subg. Anguinum are mainly mountainous, rocky places in forests, subalpine meadows and along stream banks in high mountains (Vvedenskii, 1935; Stearn, 1980; Xu and Kamelin, 2000; Fritsch and Friesen, 2002; Kawano and Nagai, 2005; Choi and Oh, 2011). Most species of subg. Anguinum share a basic chromosome number of x = 8, and 2n = 16 or 32 (Herden et al., 2016).
In this study, we describe and illustrate a new species of Allium subg. Anguinum from Ulleungdo Island, Korea, A. ulleungense H. J. Choi & N. Friesen. It is readily distinguished from its closest relatives, A. microdictyon Prokh. and A. ochotense Prokh., based on morphological, cytological, and molecular characteristics.
Taxonomic Treatment
Allium ulleungense H. J. Choi & N. Friesen, sp. nov. (Figs. 1, 2).—TYPE: Korea. Gyeongsangbuk-do: Ulleung-gun, shaded slope of Seongin-bong, 37.49766N, 130.86379E, elev. 825m, 5 Jun 2019 [fl], H. J. Choi 190605-001 (holotype: KH; isotypes: KH, KIOM).
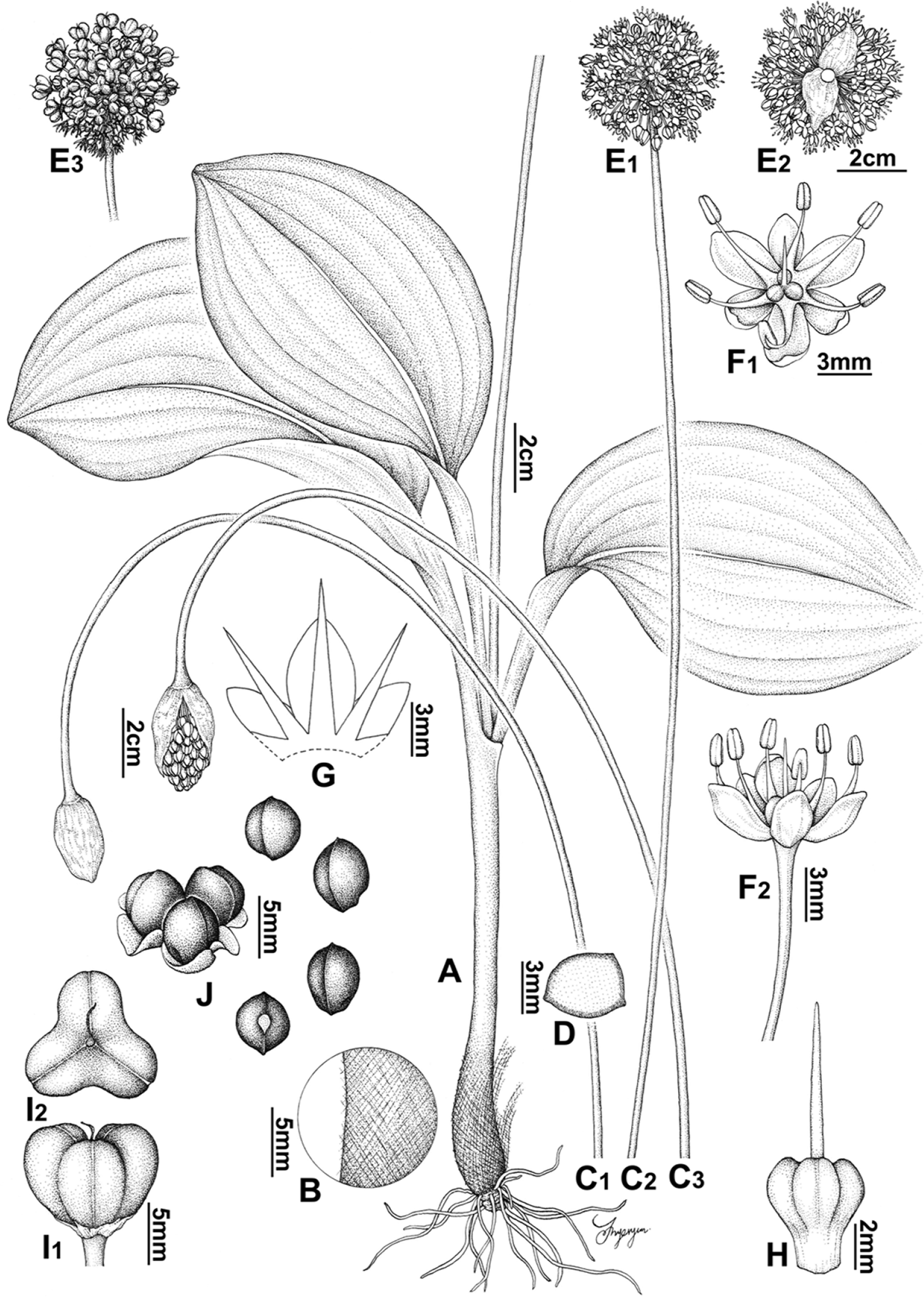
Allium ulleungense. A. Habit. B. Tunic. C. Scape. D. Shape of scape in cross section. E. Inflorescence. F. Flower. G. Tepal and filament arrangement. H. Pistil. I. Capsule. J. Seed. Illustrations by Hyeryun Jo.

Allium ulleungense. A. Habit (from the holotype). B. Inflorescence. C. Underground structure and bulb tunic (r, rhizome). D. Tepal and filament arrangement. E. Flower. F. Pistil. G. Capsule. H. Seed (B–H from Fig. 8 of Choi and Oh, 2011).
Herbs, hermaphroditic. Rhizomes condensed, oblique, 5–15 mm long. Bulbs solitary or clustered, cylindrically conical, 11.5–20 mm in diam.; tunic fibrous, reticulate, brown. Leaves 2 or 3; sheaths exposed above ground, 17–32 cm long, pale green; blade ascending, elliptic to oval, flat, 19.5–30 × 6.2–15 cm, solid in cross section, base pseudo-petiolate, apex obtuse to subrounded. Scape subterete, curved distally before flowering, solid in cross-section, 40–86 cm × 2.2–6.1 mm. Inflorescence umbellate, globose, 26.5–54 × 31–51 mm, without bulbils, 26–110 flowered; pedicels multiangular, subequal in length, 14.3–25 mm long; bracts 7.5–17 mm long. Flowers bisexual; perianth campanulate, white; inner tepals longer than outer ones, elliptic, apex obtuse, 6.0–8.5 × 3–3.7 mm; outer tepals oblong, apex obtuse, 5.7–7.2 × 1.6–1.8 mm; filaments exserted, 7.5–9.1 mm long, margin entire; anthers ellipsoid to oblong, yellowish, 2.3–2.6 mm long; ovary obconical, green or sometimes tinged reddish, 3.8–4.8 × 2.9–3.3 mm, ovules 1 per locule; style terete, exserted; stigma smooth. Capsules cordiform, trigonous, 6–6.5 × 6.5–7.5 mm. Seeds globose or nearly so, 2.6–4.1 × 2.5–3.5 mm.
Etymology: The specific epithet, “ulleungense,” is based on the name of the location, Ulleungdo Island, where Allium ulleungense was discovered.
Local name: Ul-leung-san-ma-neul (Choi and Oh, 2011).
Phenology: Flowering late May to mid-June.
Karyology: Chromosome number 2n = 2x = 16, diploid (Fig. 3) (Choi and Oh, 2011). The chromosomes range from 11.3 to 15.75 μm in length. The chromosome complement comprises five pairs (1, 3, 5, 6 and 8 of Fig. 3) of metacentric chromosomes and three pairs (2, 4 and 7 of Fig. 3) of submetacentric chromosomes based on Levan et al. (1964).
Distribution and ecology: South Korea, Ulleungdo Island, endemic (Fig. 4). Rare in natural habitats, but widely cultivated in Korea as an edible plant named ‘Myeong-i-na-mul’ or ‘San-ma-neul.’
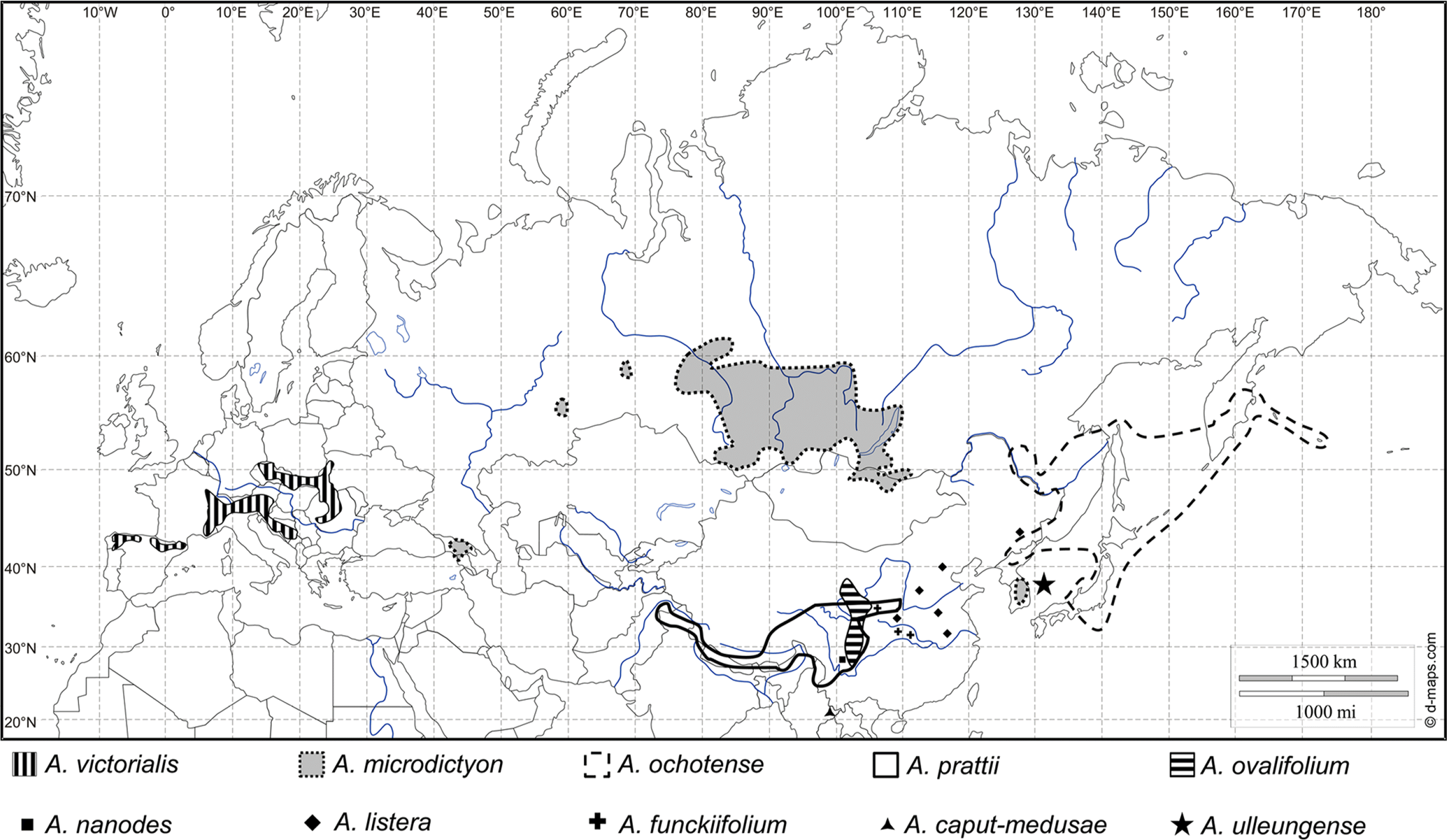
Distribution map of the species of Allium subg. Anguinum in Europe and Asia (supplemented from Fig. 3 of Herden et al., 2016).
Additional specimens examined: KOREA. Gyeongsangbuk-do: Seonginbong, Ulleungdo Isl., 18 May 2002 [fl], H.J.Choi 020056 (CBU); Seonginbong, Ulleungdo Isl., 20 May 1989 [fl], S. G. March et al. s.n. (SNUA); Seonginbong, Ulleungdo Isl., 28 Jul 1961[fr], T. B. Lee s.n. (SNUA); Albong, Ulleungdo Isl., 37°31′02.3″N, 130°51′56.5″E, elev. 420 m, 14 May 2004 [fl], S. H. Park 40940 (KH); Chusan, Ulleungdo Isl., 37°31.447′N, 130°51.236′E, 21 May 2002 [fl], S. H. Park 21587 (KH); Anpyeongjeon to Nari, Ulleungdo Isl., 3 Jun 2001 [fl], E. S. Jeon s.n. (KH); Naribunji to Seonginbong, Ulleungdo Isl., 37°29′46.5″N, 130°51′46.3″E, elev. 739 m, 12 May 2003 [fl], S. H. Park 30602 (KH).
Note: Allium ulleungense was previously identified as A. victorialis (Yu et al., 1981) or A. ochotense (Choi and Oh, 2011) in Korea, but is also morphologically similar to A. microdictyon (Choi and Oh, 2011). However, it is clearly distinguished from A. microdictyon and A. ochotense, particularly by its relatively broader leaves (mean 110 mm wide) and larger whitish perianth (Fig. 5, Table 1). Additionally, A. ulleungense is diploid (2n = 2x = 16) (Fig. 3) along with A. microdictyon (Herden et al., 2016), whereas A. ochotense has been reported to be tetraploid (2n = 4x = 32) (Kawano and Nagai, 2005; Probatova et al., 2006) or rarely pentaploid (Probatova et al., 2006). Molecular phylogenetic analyses using nuclear ITS and chloroplast markers (rps16, rbcL–atpB, rpl32–trnL) also clearly indicate that A. ulleungense is genetically distinct from other species of subg. Anguinum (Fig. 6).
Comparative photographs of inflorescences, flowers, tepal and filament arrangement, and leaf blades of Allium ulleungense (A–D), A. microdictyon (E–H), and A. ochotense (I–L).
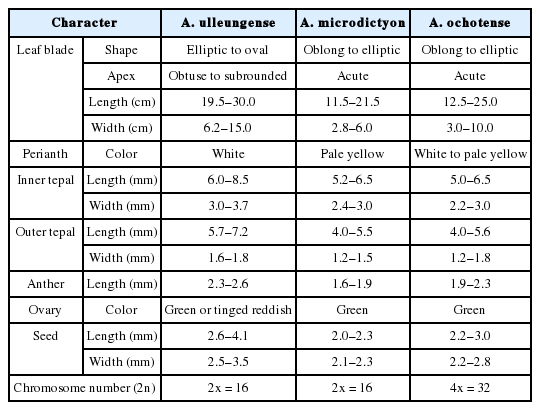
Comparison of major characters of Allium ulleungense, A. microdictyon, and A. ochotense (measurements from a minimum of 30 specimens of each).
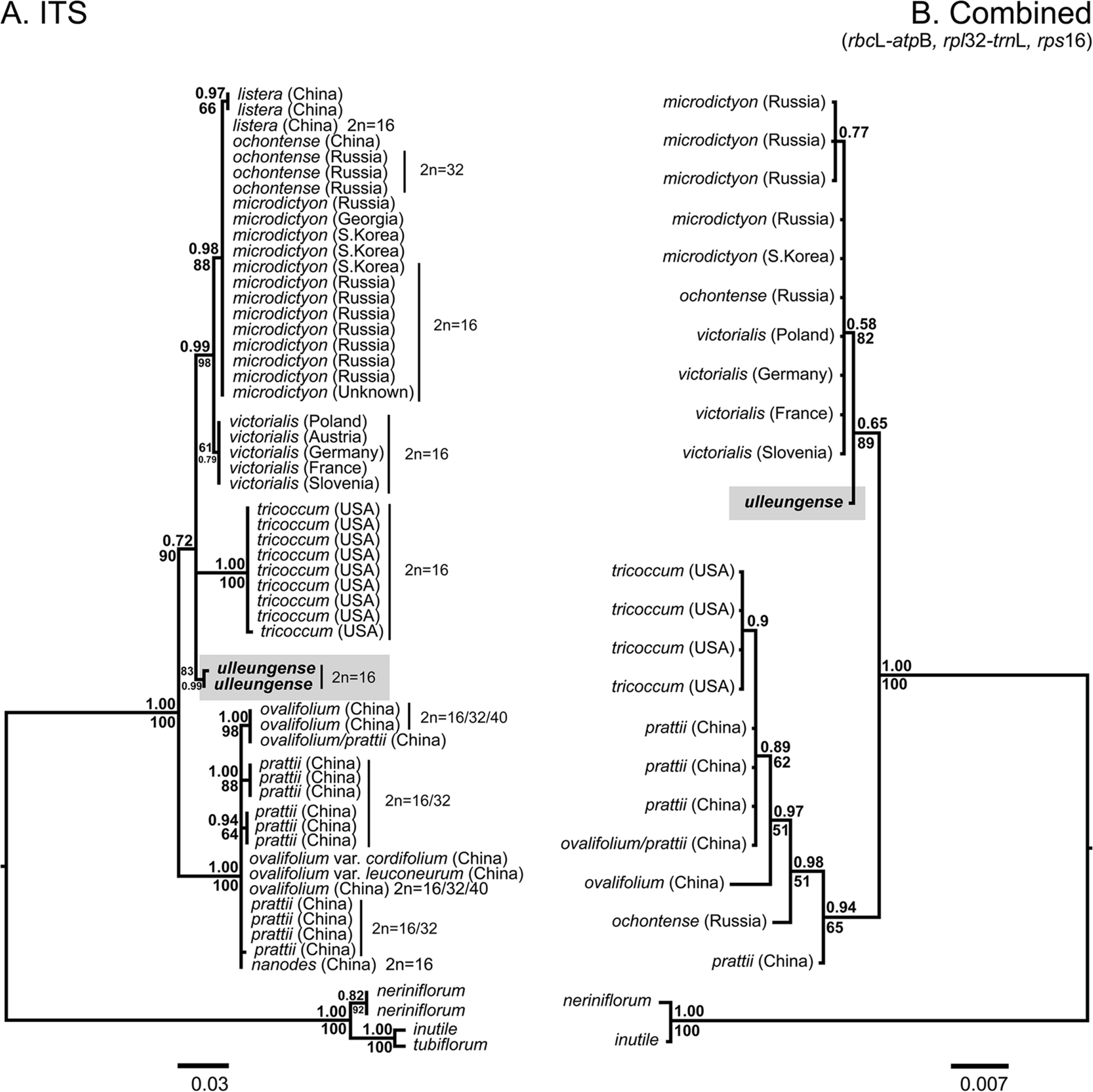
Phylogenetic trees resulting from Bayesian analyses of the internal transcribed spacer (ITS) sequences (A) and combined cpDNA sequences (B) of subgenus Anguinum. Bayesian posterior probabilities (>0.5) above branches, bootstrap support (>50%) below branches. All sequence data are from Herden et al. (2016), but accessions Gb-19 and Am165 were reidentified as Allium ulleungense in this study.
Acknowledgements
Research for this article was supported by the research project, Review on the Korean native Allium species (Project Number KNA 1-2-35, 19-3) from the Korea National Arboretum, South Korea. Two anonymous reviewers, whose comments and corrections improved the work, are also acknowledged.
Notes
ORCID: Hyeok-Jae CHOI https://orcid.org/0000-0001-6315-0071 ; Sungyu YANG https://orcid.org/0000-0001-5081-0296; Jong-Cheol YANG https://orcid.org/0000-0003-2133-2883; Nikolai FRIESEN https://orcid.org/0000-0003-3547-3257
Conflict of Interest
The authors declare that there are no conflicts of interest.