좀목포사초, 한국에서 발견된 1신종
Carex brevispicula (Cyperaceae), a new species from Korea
Article information
Abstract
사초과의 좀목포사초(Carex brevispicula G. H. Nam & G. Y. Chung)가 한국에서 처음으로 발견되었다. 좀목포사초는 수과의 중앙부가 함몰되는 점에서 큰청사초(C. chungii Z. P. Wang) 및 목포사초(C. genkaiensis Ohwi)와 유사하다. 그러나 좀목포사초는 식물체, 웅화서와 자화서가 소형이고, 자인편이 연녹색인 특징으로 큰청사초와 구분되며, 자인편과 웅인편에 까락이 있다는 점에서 목포사초와 차이를 보인다. 새로운 국명은 목포사초보다 작다는 의미로 좀목포사초로 하였고, 주요형질에 대한 기재, 도해, 사진, 분포정보 및 근연분류군과의 차이점을 검색표로 제시하였다.
Trans Abstract
A new species, Carex brevispicula G. H. Nam & G. Y. Chung (Cyperaceae), was found in Korea. Carex brevispicula is similar to the related species C. chungii Z. P. Wang and C. genkaiensis Ohwi in that its achenes are constricted in the middle part. However, C. brevispicula is distinguished from C. chungii as the plants, staminate, and pistillate spikes are shorter and its pistillate scales are pale green; C. brevispicula is distinguished from C. genkaiensis by its awned staminate and pistillate scales. The scientific name of this new species was based on the fact that its inflorescence is shorter than that of C. chungii. The corresponding Korean name, “Jom-mok-po-sa-cho,” means that the plants of this species are smaller than the “Mok-po-sa-cho” types (C. genkaiensis). We hereby provide a description of C. brevispicula, with corresponding illustrations and photographs, a distribution map, and a key of related taxa.
The genus Carex L. is the largest genus of monocots and comprises more than 2,000 species (Global Carex Group, 2015; Govaerts, 2020). These species are broadly distributed throughout the polar and temperate regions, and are sporadically found in some tropical regions (Egorova, 1999; Dai et al., 2010; Govaerts, 2020). Notably, morphological characters such as spike number, sex type, stigma number, achene shape, and appendage presence are known to be useful in the classification of Carex into subgenera and sections (Ball, 1990; Reznicek, 1990; Goetghebeur, 1998). Section Mitratae Kük. is characterized by the presence of a staminate terminal spike, 1–4 lateral spikes that are often pistillate, 2–3 stigmas, and trigonous achenes with appendages at the apex (Kükenthal, 1909; Akiyama, 1932; Ohwi, 1936; Koyama, 1961; Egorova, 1999; Oh, 2006). It is morphologically distinguished from the related sect. Rhomboidales by a smaller perigynium size (<5 mm long) and the presence of discoid-annulate or cylindrical appendages at the apex of the achene (Katsuyama, 2015). With 45–60 recognized species, the sect. Mitratae is broadly distributed across Asia and Europe, with a few species expanding to Australia, North Africa, and North America (Egorova, 1999; Tang et al., 2010). Twenty-five species of the sect. Mitratae, including Carex breviculmis R. Br., C. genkaiensis Ohwi, C. polyschoena H. Lév. & Vaniot, and C. sabynensis Less. ex Kunth, are currently recognized to be vegetated in the Korean Peninsula (Nam, 2017).
Recently, Jang et al. (2012) reported a new distribution of a sect. Mitratae member in Korea, C. kamagariensis K. Okamoto, that was distinguished from related species by its pistillate scales with long awns and constricted achenes. However, Jin (2017) considered C. kamagariensis as a synonym of C. chungii Z. P. Wang based on a morphological study. While reviewing the Carex sect. Mitratae collected in Korea, we found that the taxon from Korea commonly recognized as C. kamagariensis could be classified into two species, C. chungii and a new species. We discovered that C. chungii grows in the southern provinces of Korea and Japan, as well as in northern China (Nam et al., 2014; Jin, 2017), and is characterized by longer plants (28–58 cm), staminate spikes (14–28 mm), and pistillate spikes (14–32 mm) with brown pistillate scales. In contrast, the new species thrives throughout South Korea, except in Jejudo Island. Its height is 6–33 cm, and the lengths of the staminate and pistillate spikes are 8–17 mm and 6–14 mm, respectively, which is smaller than those of C. chungii; furthermore, the new species has pale green pistillate scales (Nam, 2017).
In this study, we describe and illustrate a new species of Carex from the sect. Mitratae that was identified in Korea, C. brevispicula G. H. Nam & G. Y. Chung. The taxonomic key and table with related taxa are provided for comparison of the main morphological characteristics and the micro-characteristics, as observed by scanning electron microscopy (SEM).
Taxonomic Treatment
Carex brevispicula G. H. Nam & G. Y. Chung, sp. nov. (Figs. 1–3).─TYPE: KOREA. Jeollabuk-do: Jinan-gun, Jwapo-ri, Pung-hyeol-laeng-cheon, 13 May 2011, G. H. Nam Cerex201 (Holotype: KB, Barcode NIBRVP291950, Isotype: KB, Barcode NIBRVP619368, NIBRVP619369).
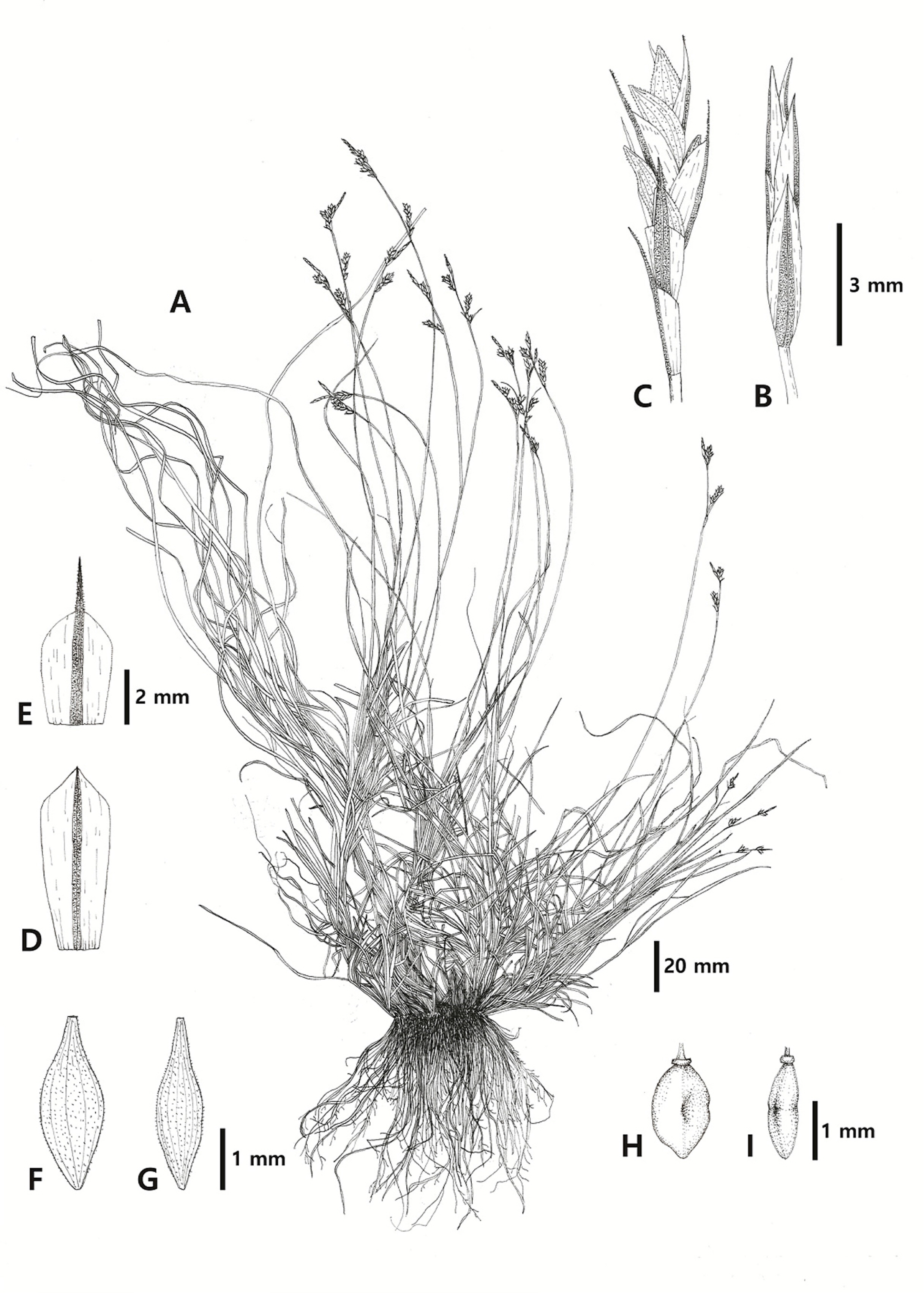
Illustrations of Carex brevispicula. A. Habit. B. Terminal spike (staminate). C. Lateral spike (pistillate). D. Staminate scale. E. Pistillate scale. F, G. Perigynium (F, abaxial view; G, side view). H, I. Achene (H, abaxial view; I, side view).

Photographs of Carex brevispicula. A, B. Habit (A, flower; B, fruit). C. Basal structure. D. Inflorescence. E. Staminate scale. F. Pistillate scale. G. Perigynium. H. Achene.
Korean name: Jom-mok-po-sa-cho (좀목포사초).
Perennial herbs, 6.6–33.0 cm tall, cespitose. Culms 6.4–29.3 cm long. Basal sheaths 0.6–1.4 cm long, brown, veined, fibrillose. Leaves as long as culms, blades 6.7–29.7 cm × 0.8–2.3 mm, light green, soft. Bract sheaths 2.1–5.0 mm long; bracts 2–3, 5.0–28.0 × 0.1–0.5 mm, setaceous, shorter than spikes. Inflorescences with 3–4 spikes, densely racemiform, 1.9–5.6 cm long. Terminal peduncles 1.6–9.9 mm long; terminal spike 1, staminate, linear cylindrical, 8.0–16.9 × 0.9–1.7 mm. Staminate scales pale green, narrowly obovate, 3.3–4.5 × 1.0–1.5 mm, round at apex or shortly awned. Lateral peduncles 0–2.4 mm long; lateral spikes 2–3, pistillate, erect, narrowly cylindrical, 5.9–14.4 × 1.8–2.4 mm. Pistillate scales pale green, elliptically ovate, 1.8–2.5 × 0.9–1.4 mm, long awned at apex, 0.5–1.7 mm long. Stigmas 3. Perigynia as long as pistillate scales, 1.9–2.2 × 0.9–1.4 mm, distinctly 5–6 veined, pubescent, beaks 0.5–1.7 mm, mouth notched. Achenes tightly enveloped by perigynia, obovoid, 1.8–2.3 × 0.8–1.0 mm, slightly constricted in the middle, discoid–annulate at apex.
Taxonomic note: The new species is similar to the related species C. breviculmis R. Br., C. chungii Z. P. Wang, C. genkaiensis Ohwi and C. mitrata var. aristata Ohwi. However, it has achenes that are constricted in the middle, distinguishing it from C. breviculmis and C. mitrata var. aristata (Fig. 4). Furthermore, its pale green pistillate spikes with long awns descriminate it from C. chungii and C. genkaiensis (Table 1). In addition, among the above mentioned species, papillae on the perigynium surface were only observed in C. chungii (Fig. 5).

Scanning electron microscopy photographs of achene shapes. A. Carex brevispicula. B. Carex breviculmis. C. Carex chungii. D. Carex genkaiensis. E. Carex mitrata var. aristata. The arrows indicate constriction in the middle part of the achene. 1, shortly cylindrical at achene apexes; 2, annulated at achene apexes.
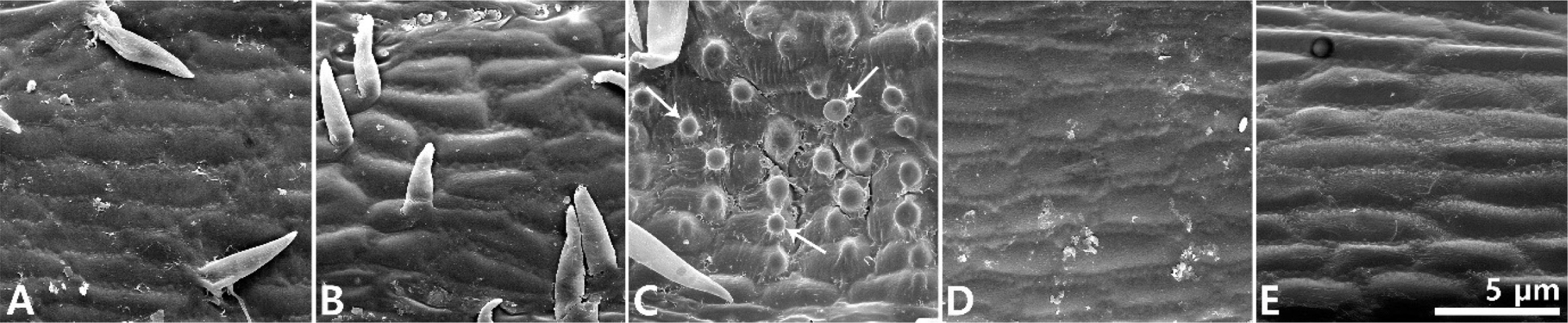
Scanning electron microscopy photographs of perigynium epidermis. A. Carex brevispicula. B. Carex breviculmis. C. Carex chungii. D. Carex genkaiensis. E. Carex mitrata var. aristata. The arrows indicate the papilla.
Distribution and habitat: Carex brevispicula is distributed throughout South Korea; Gangwon-do to the islands of the southern provinces, except in Jeju-do Island (Fig. 6). Notably, We could not examine the distribution of this species in North Korea. This species perennates on mountain slopes, under half-shadow conditions, and mainly prefers the rocky environments.
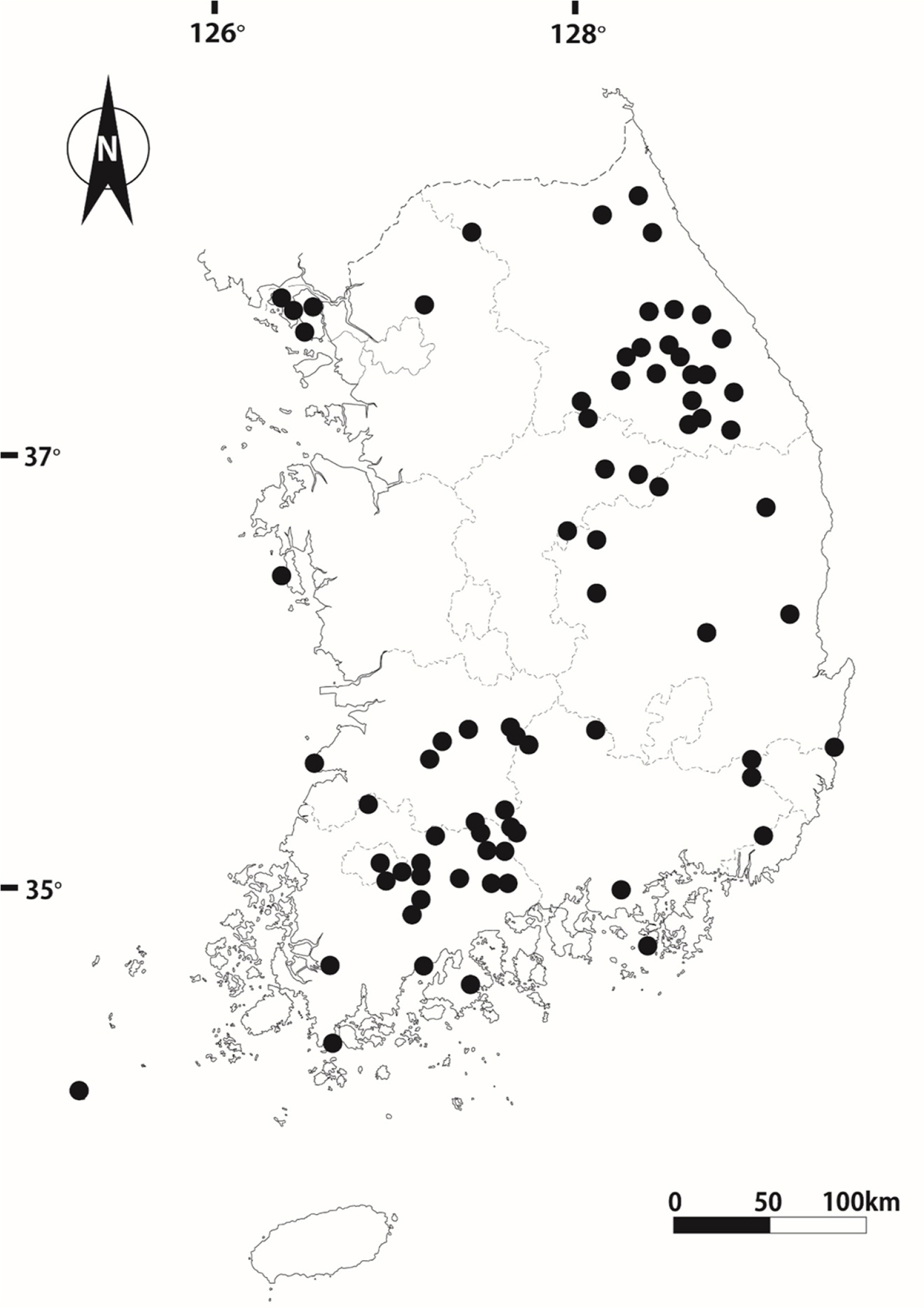
Distribution of Carex brevispicula in South Korea. Vouchers refer to Nam (2017).
Phenology: C. brevispicula flowers from March to April and fruits from May and June.
Etymology: The specific epithet “brevispicula” refers to the fact that the inflorescence of this species is shorter than that of C. chungii Z. P. Wang. For the corresponding Korean name, we used the existing name “Jom-mok-po-sa-cho” (Jang et al., 2012), because C. kamagariensis, reported by Jang et al. (2012) as an unrecorded species in Korea, is this newly identified species. The name means that the plants of this species are smaller than those of “Mok-po-sa-cho” (C. genkaiensis Ohwi). In addition, we suggest that the Korean name for C. chungii (accepted name of C. kamagariensis) be “Keun-chung-sa-cho,” in accordance with Nam et al. (2014).
Conservation status: This species is widely distributed in South Korea. Therefore, no special conservation measures are required at present.
Additional specimens examined: KOREA. Gangwon-do: Jeongseon-gun, Hambaeksan Mt., 4 Jun 2013, G. H. Nam NGH13-196, KB (NIBRVP435122); Pyeongchang-gun, Woljeongsa, 2 Jun 2012, J. H. Kim Carex-Kim130, KB (NIBRVP362158). Gyeongsangbuk-do: Cheongsong-gun, Juwangsan Mt., Baengnyeonam, 22 Apr 2017, H. D. Jang 508, KSM. Gyeongsangnam-do: Hamyang-gun, Jirisan Mt., 6 Jun 2010, G. H. Nam & J. H. Do Carex35, KB (NIBRVP291986); Miryang-si, Gajisan Mt., 7 May 2011, G. H. Nam Carex173, KB (NIBRVP291922). Jeollanam-do: Boseong-gun, Cheonseongsan Mt., 24 Apr 2010, J. H. Kim & Y. H. Cho Kim110769, KB (NIBRVP353066); Gurye-gun, Jirisan Mt., Nogodan, 6 Jun 2006, J. H. Kim KJH162, KB (NIBRVP274808); J. H. Kim KJH163, KB (NIBRVP274809). Seoul: Buramsan Mt., 6 May 2014, C. S. Lee Leecs1354, KB (NIBRVP505672).
Key to Carex brevispicula and related taxa
Achene angles not contracted, achenes excavated at faces
2. Terminal spike pale brown; lateral spikes linear ··········· ····················································· C. mitrata var. aristata
2. Terminal spike pale green; lateral spikes short cylindrical ···································································· C. breviculmis
1. Achene angles contracted in middle part
3. Pistillate scale apexes acute or shortly awned; achene apexes shortly cylindrical ······················· C. genkaiensis
3. Pistillate scale apexes long awned; achene apexes shortly annulated
4. Pistillate scales pale brown; perigynium surface papillate; plant 28–58 cm and lateral spikes 14–33 mm ························································ C. chungii
4. Pistillate scales pale green; perigynium surface smooth; plant less than 33 cm and lateral spikes less than 15 mm ······································· C. brevispicula
Acknowledgements
This work was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202007103).
Notes
Conflict of Interest
The authors declare that there are no conflicts of interest.


