Chromosome number of Carex brevispicula (Cyperaceae), a sedge endemic to Korea
Article information
Abstract
Carex brevispicula (Cyperaceae) is endemic to Korea and is characterized by constricted achenes, short lateral spikes, and awned staminate and pistillate scales. The species classified in sect. Mitratae occurs throughout South Korea, perennating on mountains and/or rocky slopes under half shadow conditions. Meiotic chromosomes of the species were examined in this study, in which 33 meiotic cells from seven populations were found to be less than 2 μm long with non-constricted chromosomes (n = 34II). The stable chromosome number may be related to the narrow geographical distribution and/or distinct achene morphology. Further investigations of the distribution, morphological character variation, and chromosome characteristics should be conducted with closely related taxa to understand the derivation of the species and its endemism in Korea.
INTRODUCTION
Carex brevispicula G. H. Nam & G. Y. Chung (Cyperaceae) is newly described from Korea in sect. Mitratae Kükenthal (Nam et al., 2020). The species has morphological similarity with C. kamagarienesis K. Okamoto, C. chungii Z. P. Wang, and C. breviculmis R. Br., sharing constricted achenes. However, the new species is characterized by short heights, short cylindrical achene apexes, pale green pistillate scales, and awned staminate and pistillate scales. Carex brevispicula occurs throughout South Korea, habituating on mountain slopes or rocky slopes of half shadow conditions (Fig. 1) (Nam et al., 2020). To clarify distribution areas of the species, Asian regions such as China and Japan, where morphologically similar taxa grow, should be investigated (Hoshino et al., 2011; Nam, 2017).
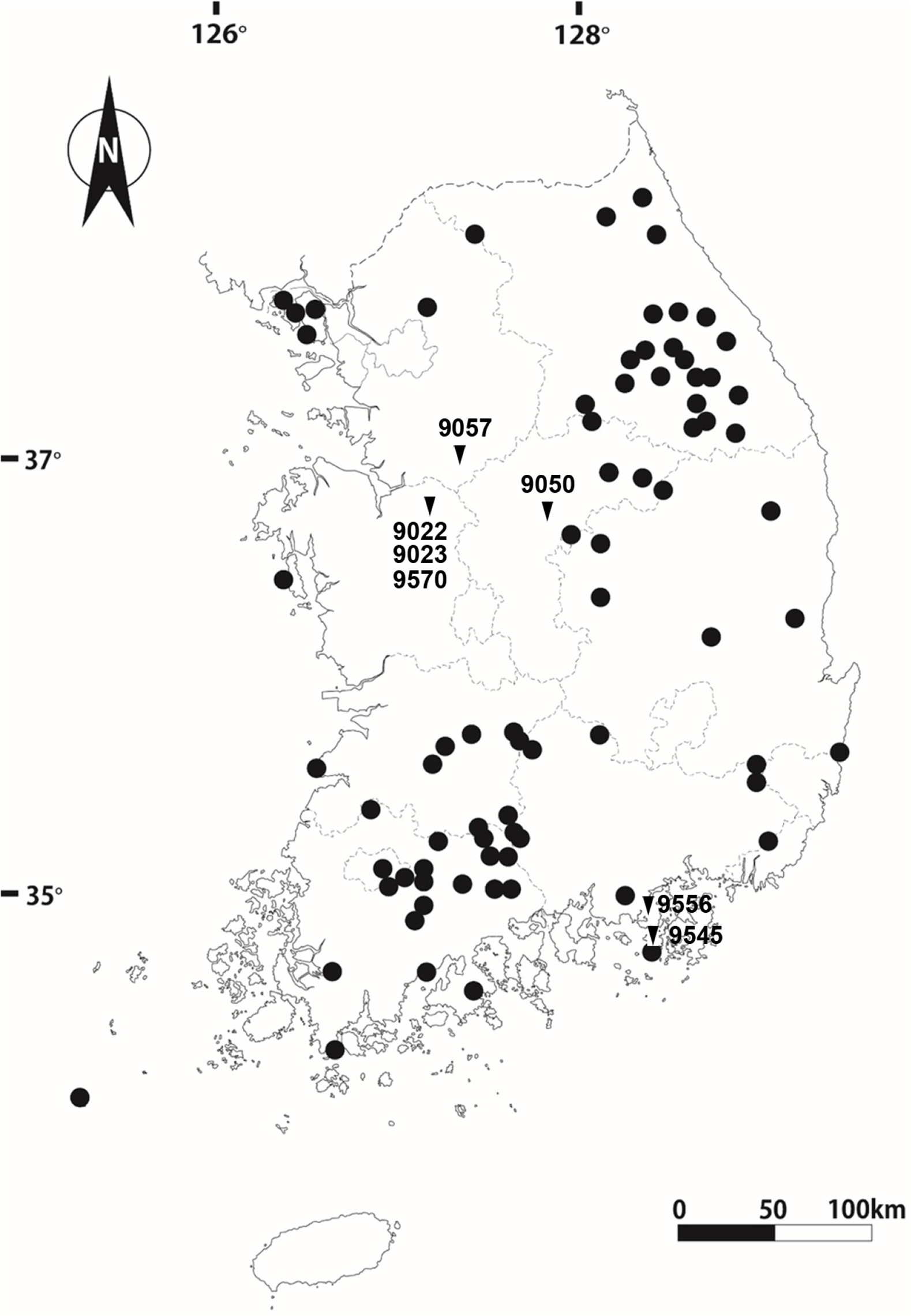
Distribution and sample sites of Carex brevispicula. Circles refer distribution sites, and inverted triangles (with voucher numbers) indicate chromosome sample sites in the present study. The map is adopted from Nam et al. (2020).
Carex L. is the largest genus among vascular plants in the temperate zone (more than 2,000 taxa worldwide) and also the largest genus in Korean flora (about 157 species) (Oh, 2007; Global Carex Group, 2015). High species richness of the genus has been explained by holocentric chromosomes, which miss constricted centromeres during cell divisions (Luceño and Castroviejo, 1991; Roalson, 2008). Due to the holocentric feature, chromosome numbers in the genus exhibits broad variations from n = 6 to n = 66 (Tanaka, 1949; Hipp et al., 2009). Furthermore, variations in chromosome numbers have been reported within species and/or individuals (Rothrock and Reznicek, 1996; Hipp et al., 2010; Chung et al., 2011).
Sect. Mitratae is the largest section in Korean Carex with 28 taxa (45–60 taxa worldwide), and haploid chromosome numbers ranging from n = 10 (C. blenpharicarpa Franch.) to n = 40 (C. stenostachys Franch. & Sav.) (Nam, 2017; Chung and Im, 2020). With the exception of three species, the most species in the section exhibit variation in chromosome numbers. Carex multifolia Ohwi exhibits the broadest range of variation, from 2n = 30 to from 2n = 70 (30, 60, 64, 65, 66, 70) (Chung and Im, 2020). Variation in chromosome numbers might reflect genetic diversity within and/or among individuals in a taxon. Luceño and Castroviejo (1991) and Hipp et al. (2010) proposed positive correlations between chromosome number and genetic diversity as well as chromosome number and geographic distance in Carex.
In the present study, meiotic chromosome numbers of C. brevispicula were examined to evaluate the variation in chromosome numbers and sizes, sampled from multiple populations of natural habitats in Korea.
MATERIALS AND METHODS
Meiotic chromosomes of the species were examined following the methods for Carex in Chung and Chung (2021). During early spring in 2022 and 2023, immature staminate spikes were sampled (Table 1) and fixed from seven populations covering distribution areas (Fig. 1). A mixture of methanol, chloroform, and propionic acid (6:3:2) fixed the immature spikes and then stored in 70% ethanol (Rothrock and Reznicek, 1996). The anthers were squashed in 1 or 2% acetic-orcein and observed at 1,000× magnification (Nikon Eclipse 50i, Nikon, Tokyo, Japan). To ascertain chromosome numbers and variation ranges, more than two meiotic division cells per individual were observed, analyzed, and photographed. Taxon identification followed Nam et al. (2020), and voucher specimens were stored at Korea National Institute of Biological Resources Herbarium (KB).
RESULTS AND DISCUSSION
From seven populations 33 meiotic cells were observed, and chromosome numbers were analyzed. Results include chromosome numbers as n = 34II, sizes are less than 2 μm long, and constricted centromeres are not visible (Fig. 2). The chromosomes seem to be small-sized holocentric. Unlike most species in the sect. Mitratae, the species exhibits consistent chromosome numbers. Similar phenomenon has been reported from sect. Ovales Kunth, the most species rich section with more than 80 taxa in North America (Mastrogiuseppe et al., 2002). Carex scoparia var. scoparia exhibits broad variation in chromosome numbers from 2n = 62 to 2n = 68, but chromosome number of C. scoparia Schkuhr ex Willdenow var. tessellate Fernald & Wiegand is stable with 2n = 66 (Chung et al., 2011, 2012). In addition, C. scoparia distribute widely in Northeast America whereas C. scoparia var. tessellate occurs narrowly in New Brunswick (Canada) and Maine (USA) (Mastrogiuseppe et al., 2002).

Photomicrographs of meiotic chromosomes of Carex brevispicula (n = 34II). A. Chung 9022. B. Chung 9023. C, D. Chung 9050. E. Chung 9057. F, G. Chung 9545. H. Chung 9556. I–L. Chung 9570. Scale bars = 10 μm.
In the recent molecular investigation, C. brevispicula is sister to C. breviculmis, which has various chromosome numbers from n = 32 to n = 35 in Korean populations, and 2n = 64, 68, 70, 72, 74 in Japanese populations (Tanaka, 1939; Hoshino, 1981; Ohkawa and Yokota, 1998; Nam, 2017; Chung and Im, 2020; Chung and Chung, 2021). The clade, which C. brevispicula belongs to, includes taxa with short peduncles and awned male or female scales (C. breviculmis complex) (Nam, 2017), and the most morphologically similar taxa with C. brevispicula are also supported by molecular data (nrITS, nrETS, and matK) as closely related species (C. genkaiensis, C. mitrata var. aristata, C. kamagariensis, and C. breviculmis) (Nam, 2017). However, C. rugata, which shares distinct characteristics of constricted achenes with C. brevispicula, is placed in the C. sabynensis complex clade (Nam, 2017). Except for C. breviculmis, chromosome numbers of the constricted achene possessing taxa have not been investigated. Evaluation of chromosome data for those taxa in a robust phylogenetic hypothesis will provide new insights on morphological and cytological character evolution.
Carex brevispicula, a Korean endemic sedge, has stable chromosome number of n = 34II. The chromosome feature might be related to narrow geographic distribution and/or morphological characters such as constricted achenes. Further investigation of distribution, morphological character variation, and chromosome characteristics should be conducted with closely related taxa to understand derivation of the species and endemism in Korea.
Acknowledgements
We thank Tomomi Masaki (Okayama University of Science) for critical suggestions on original data analyses. The present research was partially supported by National Research Foundation (NRF-2021R1I1A3060260).
Notes
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.