Isoёtes L. is a perennial emergent hydrophyte belonging to Isoёtaceae, most of which grow in submerged places at least once in their lifetime (Taylor and Hickey, 1992). It is known that approximately 150 species, or up to 350 species of Isoёtes, are distributed throughout the world (Taylor and Hickey, 1992; Hickey et al., 2003). Recently, however, Troia et al. (2017) classified Isoёtes plants worldwide into 192 taxa consisting of 183 species, seven subspecies, and two varieties.
Isoёtes has attained a distinct phylogenetic position in vascular plants since it first appeared in the Paleozoic Devonian (Taylor and Hickey, 1992; Pigg, 2001). Lycopodiophyta (Lycopodiaceae, Selaginellaceae, Isoёtaceae), including Isoёtes, are clearly differentiated from other monilophytes and spermatophytes due to their distinct structures of vascular bundles and lycophylls (Pryer et al., 2001). According to the molecular phylogeny of vascular plants, Lycopodiophyta is also considered to be a sister group of all other vascular plants (Smith et al., 2006). In Lycopodiophyta, Isoёtes is distinguished from other lycophytes due to the presence of ligules and heterospory features, and it forms a sister group with Selaginellaceae (Taylor et al., 1993; Yatsentyuk et al., 2001).
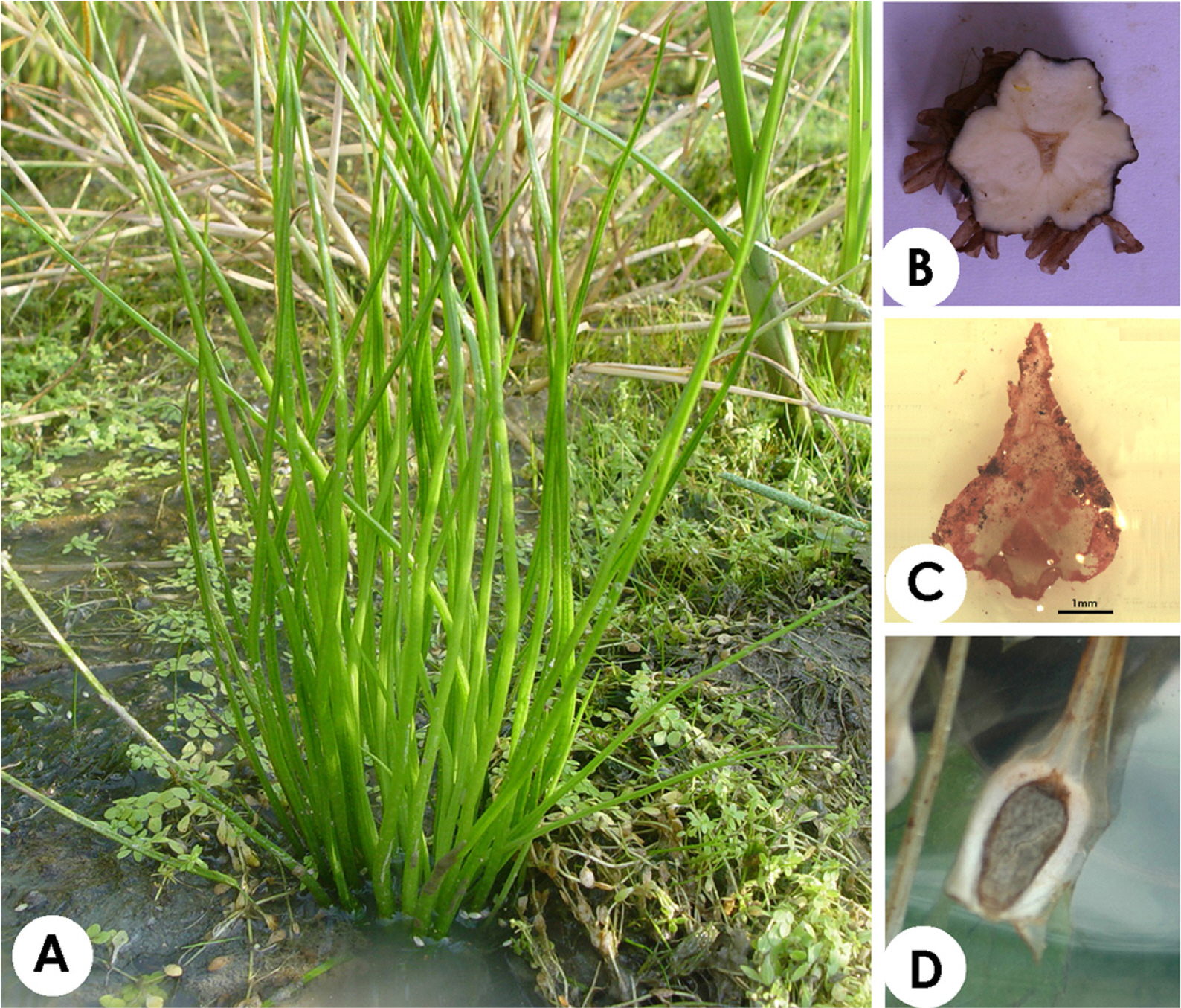
The morphologies of Isoёtes species have a very simple characteristic by which elongated sporophylls are arranged in a 2–3-lobed corm (Pfeiffer, 1922; Chung and Choi, 1986; Hickey, 1986) (Fig. 1A, B). The ligule (Fig. 1C) at the top of the sporangium protects the sporangium from the beginning of plant growth until the maturation of the spores (Sharma and Singh, 1984). Megasporangium includes megaspores and microsporangia with microspores located inside the megaphyll and microphyll bases, respectively (Fig. 1D). Due to the simple external morphology of Isoёtes species, identifying taxonomic limitations or analyzing phylogenetic relationships have remained difficult.
The surface ornamentation of megaspore in Isoёtes has traditionally been used as an important trait-delimiting sections (Pfeiffer, 1922; Fuchs-Eckert, 1981; Hickey, 1986). In addition, identification of Isoёtes species largely rests on megaspore and microspore ornamentation. Thus, they are used as the main diagnostic characters when describing new species (Wang et al., 2002; Choi et al., 2008; Kim et al., 2010a). Although the surface ornamentation of the spores is useful for identifying species, it does not reflect the phylogeny of Isoёtes at all. For example, I. echinospora complex that shows echinate ornamentation of megaspore, did not form a monophyletic group including all species of the complex (Kim and Choi, 2016). Isoёtes japonica distributed in Japan and I. durieui distributed in Turkey along the Mediterranean coast and in France are all the reticulate type. However, in previous molecular phylogenetic analyses, these two species show a distant relationship (Larsén and Rydin, 2016; Kim and Choi, 2016). Thus, the morphological features and similarity of the spores in Isoёtes are interpreted as a result of convergent evolution. Recently, a variety of molecular markers have been used to identify Isoёtes species and to assess phylogenetic relationships (Taylor et al., 2004; Hoot et al., 2006; Kim et al., 2009a, 2010b).
In East Asia, a total of 12 species of Isoёtes were reported, with four species in China (I. sinensis, I. yunguiensis, I. hypsophila, I. orientalis) and one in Taiwan (I. taiwanensis), five in Japan (I. asiatica, I. japonica, I. ×michinokuana [=I. japonica × I. pseudojaponica], I. pseudojaponica, I. sinensis), and four in Korea (I. japonica, I. coreana, I. jejuensis, I. hallasanensis) (Table 1; Palmer, 1927; DeVol, 1972; Chung and Choi, 1986; Takamiya et al. 2002; Lee, 2003; Liu et al., 2005; Choi et al., 2008). As in other regions, the number of lobes of a corm, the sizes and surface structures of megaspores and microspores, and the number of chromosomes have all been used as traits to identify East Asian Isoёtes species (Table 1) (Jung et al., 2009). For example, I. asiatica, which grows in Hokkaido, Japan, is distinguishable from other Isoёtes species in East Asia through its two lobes on the corm. Korean endemic, I. coreana is similar to I. sinensis in that it has cristate megaspores and echinate microspores but differs from this species in terms of the number of chromosomes (Table 1).
Most of the East Asian Isoёtes species are endangered species, and various studies have been carried out at the population level to establish their conservation plants because they are endemic species. Extensive research has been carried out, including studies on the growth environment for the preservation and restoration of endangered species (Wang et al., 2005) as well as studies involving population genetic diversity analyses using various molecular markers such as random amplification of polymorphic DNA, amplified fragment length polymorphism (AFLP), and nucleotide and chloroplast DNA sequences (Kang et al., 2005; Kim et al., 2008, 2009b; Jung et al., 2014). Some of the studies have also been focused on speciation mechanisms of parental species via polyploidization (Kim et al., 2010b), and addressed their phylogenetic relationships and biogeographical origins (Kim and Choi, 2016).
In this paper, we intend to review the phylogenetic relationships among Isoёtes species in East Asia and present their biogeographical origins. We also propose directions for future research based on the phylogenetic relationships and the results of biogeographical studies of East Asian Isoёtes species that have been conducted so far.
Phylogeny of East Asian Isoёtes
Isoёtes species are distributed throughout the world and grow in a variety of habitats, such as aquatic, semi-aquatic, and terrestrial environments. Although they have a long evolutionary history, considerable difficulty has arisen when studying phylogenetic relationships using morphological features due to the simplicity, convergent evolution, and speciation by polyploidization associated with these plants (Hickey et al., 1989; Hoot et al., 2004; Kim et al., 2010b). In addition, the Isoёtes taxa are highly endemic due to limited local distributions and obtaining samples can be difficult because they are endangered plants with small populations (Choi et al., 2008; Jung et al., 2009, 2013). Therefore, studies are underway to delimit Isoёtes species taxonomically using various molecular markers or to analyze the phylogenetic relationships among the species (Taylor et al., 2004; Hoot et al., 2006; Kim et al., 2009a, 2010b). In particular, species-specific molecular markers are useful for identifying the taxonomic boundaries of Isoёtes species that contain many endangered plants because they can be used to identify species or to elucidate phylogenetic relationships among the lineages using young or small samples.
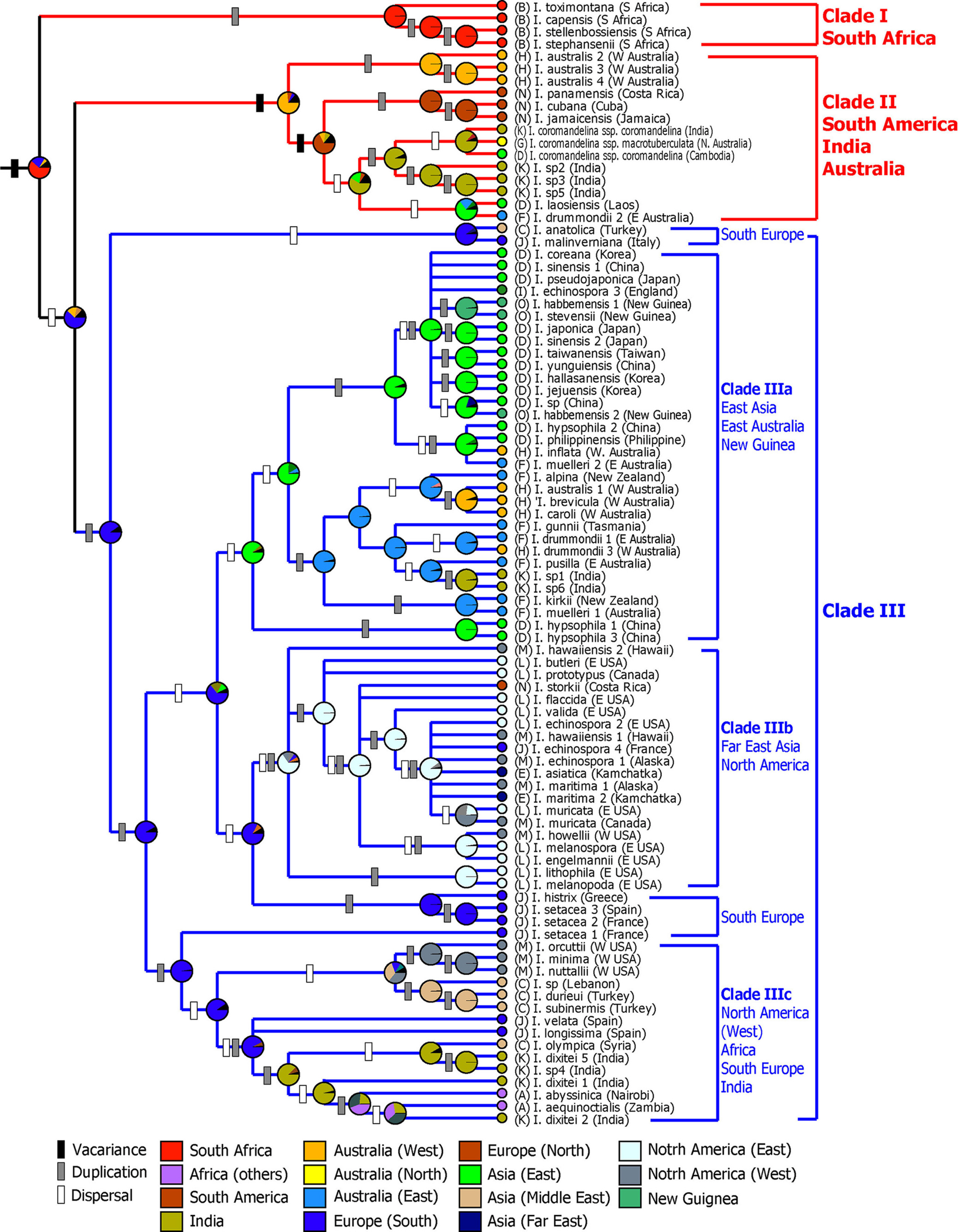
Molecular phylogenetic studies using nuclear ribosomal internal transcribed spacer (ITS) sequences have shown that the Isoёtes species divided into three major clades (Fig. 2): (1) The South African species (I. toximontana, I. capensis, I. stellenbossiensis, I. stephansenii) form a monophyletic group (clade I in Fig. 2) and sister to the group consisting of the rest of the Isoёtes species. (2) Species distributed in South America (I. panamensis, I. cubana, I. jamaicensis) form a clade with Indian species (I. coromandelina var. coromandelina) and some species in Australia and Southeast Asia (I. australis, I. coromandelina var. macrotuberculata, I. laosiensis) (clade II). (3) The remaining Isoёtes species also form a clade (clade III). Within Clade III, African species form a clade with some species in southern Europe, India (clade IIIc). In particular, the East Asian taxa are closely related to the species in eastern Australia (clade IIIa), forming a sister group with North American species (clade IIIb).
Studies of the Isoёtes in East Asia have been relatively active. Nuclear and chloroplast DNA sequences and AFLP markers have been used to reconstruct the phylogenetic relationships and for parental species identification of the polyploid species in the East Asian Isoёtes species (Taylor et al., 2004; Kim et al., 2009a, 2009b, 2010b, Kim and Choi, 2016). The East Asian Isoёtes species are divided into two clades: a clade containing I. asiatica and a clade containing the remaining East Asian Isoёtes species (Kim et al., 2009a, 2010a). In other words, I. asiatica is closely related to Isoёtes species (I. echinospora, I. maritima) in the Russian Far East and in North America, specifically in Alaska (Kim et al., 2009a), forming a sister group with the Isoёtes species distributed in Papua New Guinea and Australia (Kim et al., 2010b). Therefore, East Asian Isoёtes species can be considered to have at least two independent evolutionary histories.
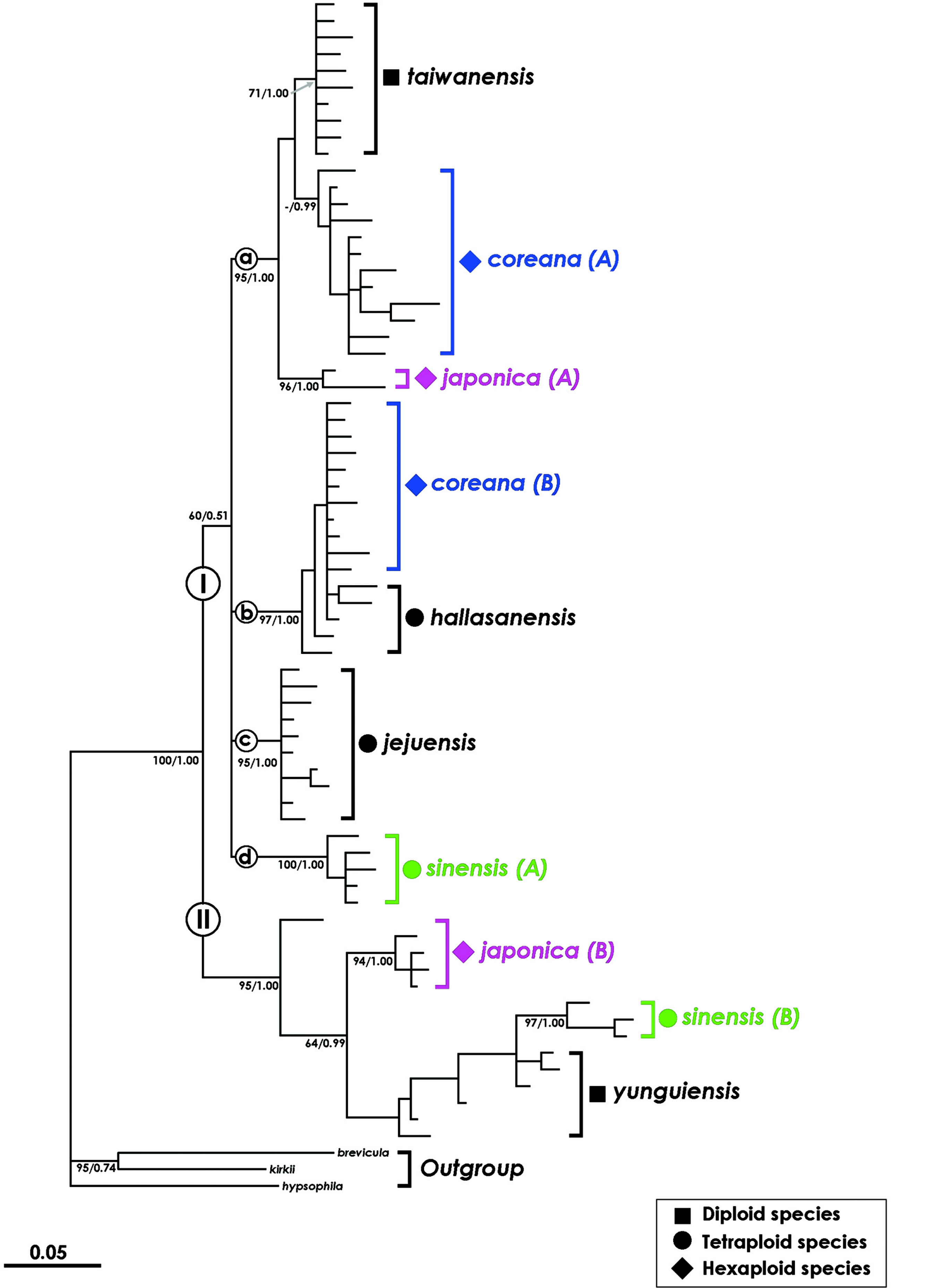
Meanwhile, a phylogenetic analysis using the nuclear LEAFY gene revealed a parental species of the East Asian Isoёtes species excluding I. asiatica (Kim et al., 2010b). In this phylogenetic tree (Fig. 3), East Asian Isoёtes plants were divided into two clades. Of these, I. coreana, I. japonica, and I. sinensis were divided into two groups (A and B), respectively, and none of the groups formed a monophyletic group. The first clade included I. taiwanensis, I. coreana (A, B), I. jejuensis, I. hallasanensis, I. coreana (A), and I. sinensis (A). The second clade contained I. yunguiensis, I. coreana (B), and I. sinensis (B). Diploid species, I. taiwanensis and I. yunguiensis, were located in both clades. In the first clade, I. coreana (A) and I. coreana (B) were clustered with I. taiwanensis and I. hallasanensis, respectively. Isoёtes japonica (A) showed a close relationship with I. taiwanensis, whereas I. japonica (B) showed a close relationship with I. sinensis (B). This relationship is supported by phylogenetic analyses using chloroplast DNA (Kim et al., 2010b). Therefore, the parental species of I. coreana (6 ×) in South Korea were I. taiwanensis (2 ×) and I. hallasanensis (4 ×), while those of I. japonica (6 ×) in Japan were I. taiwanensis and I. sinensis (4 ×) according to the tree (Fig. 4).
Thus far, the phylogeny of Isoёtes species in East Asia has been studied in detail, but there has been little research on taxa in Southeast Asia, India and Australia. Hoot et al. (2006) included only two of the taxa (I. coromandelina, I. australis) distributed in India and Western Australia in their phylogenetic study, and they did not include Isoёtes species in Southeast Asia. Therefore, there is almost no phylogenetic relationship to Isoёtes in these areas. Recently, new species of Isoёtes have been described among the flora of Southeast Asia and India (Shukla et al., 2005; Kim et al., 2010a; Jung et al., 2013). Among the taxa growing in the Southeast Asian region, I. philippinensis growing in the Philippines is closely related to the species in East Asia, and I. laosiensis reported in Laos is linked to species distributed in India and in Australia (Fig. 2). Southeast Asia appears to have species from different lineages, and additional phylogenetic studies, including those focusing on taxa from India, Southeast Asia, and northwest Australia, may be necessary.
Phytogeographical origin of the East Asian Isoёtes
Isoёtes plants are among the most widely distributed species in the world and have the highest species diversity (45 species) in many areas, including the western parts of Brazil and other areas in South America (Troia et al., 2017). In addition, it was found that species diversity is relatively high in eastern North America (26 species), Southern Europe (19 species), Australia (16 species) and South Africa (14 species). Molecular clocks and phytogeographical analyses using DNA sequences have revealed that Isoёtes diverged from Selaginellaceae during the Devonian period (mean=375 million years ago [mya]), and the main crown group of Isoёtes diverged during the Jurassic period (mean=147 mya) (Larsén and Rydin, 2016; Pereira et al., 2017). Vicariance has been found to play an important role in the initial diversification of Isoёtes species, as the taxa from Australia, South America, India, and Africa included on the Gondwana landmass formed a monophyletic group (Pereira et al., 2017). On the other hand, it has been suggested that Isoёtes species distributed in North America and East Asia were formed by long-distance dispersal by means of migratory birds after the Cenozoic period (Taylor and Hickey, 1992; Liu et al., 2004).
Two hypotheses have been proposed regarding the biogeographic history of East Asian Isoёtes plants, one based on an analysis of the cytological characteristics and the other based on molecular phylogenetic research. Liu et al. (2004), based on the number of chromosomes and on fossil data, suggested that Chinese Isoёtes plants migrated eastward from the Qinghai-Tibet region of China (I. hypsophila [2×] - I. yunguiensis [2×] - I. taiwanensis [2×] - I. sinensis [4×]) and migrated from the highlands to the lowlands through the water system. On the other hand, Hoot et al. (2006) suggested that the Isoёtes plants distributed in East Asia/Australia form a monophyletic group based on their nucleotide and chloroplast DNA sequences and that they were spread to East Asia through Australia and New Guinea by migratory birds. However, this study not only performed a DNA analysis with limited samples but also had a very low resolution of the phylogenetic relationships among the lineages in the East Asia/Australia clades. They also noted that it is necessary to carry out a more in-depth phylogenetic analysis of East Asian Isoёtes using more taxa and high-resolution molecular markers. Recently, Kim and Choi (2016) undertook phylogenetic studies and biogeographical studies of Isoёtes plants in East Asia, Australia, Papua New Guinea, the Russian Far East, and North America using nucleotide ITS and three chloroplast DNA (atpB-rbcL, trnL, trnS-psbC) sequences. As mentioned above, the East Asian Isoёtes species are divided into two major lineages: (1) northern Asian species and (2) the remaining East Asian Isoёtes (Clade III a and b in Fig. 2, respectively). The northern Asian Isoёtes in the Russian Far East and in Siberia are closely related to Isoёtes plants in western North America and Alaska. Therefore, it has been suggested that I. asiatica distributed in Hokkaido migrated through the Bering land bridge from the Alaska region of North America during late Miocene (mean = 11.2 mya). The rest of the East Asian Isoёtes species were closely related to the Isoёtes in Papua New Guinea and Australia suggesting that they have migrated through longdistance dispersal up to the late Oligocene (mean = 25.2 mya). Therefore, East Asian Isoёtes species have differentiated more recently than the Gondwana species (Figs. 2 and 5). The disjunction distribution pattern between the northern hemisphere and the southern hemisphere was largely accounted for by migrations between North America and South America and between Europe and Africa at various times during the Cenozoic Tertiary, while the migration path between Australia and East Asia was relatively less supported (Raven and Axelrod, 1974; Iturralde-Vinent and MacPhee, 1999; McLoughlin, 2001; Morley, 2003; Nie et al., 2012). However, biogeographic studies of the East Asian Isoёtes suggest the importance of migration routes between Australia and East Asia (Hoot et al., 2006; Kim and Choi, 2016). It will be necessary to confirm the dispersion mechanism of East Asian Isoёtes by comparing the results of biogeographical studies between Australia and East Asia with the migration routes of migratory birds (Fig. 5).
Suggestions for future research on Isoёtes
Isoёtes species are mostly endangered aquatic plants with limited distributions and small populations, and the establishment of conservation and restoration plans is crucial to secure their biodiversity and genetic diversity. In order to establish a conservation and restoration plan, a clear taxonomic identification of the taxa to be preserved and restored is essential. In addition, with regard to rare plants that are in danger of extinction, it is necessary to develop species-specific molecular markers that can identify species even when only very few samples exist. It is also necessary to grasp evolutionary patterns and migration routes by comparing and analyzing the relationships and geographical distributions among species or of individual species through phylogenetic and biogeographical studies. Phylogenetic studies should be preceded by the establishment of conservation and restoration plans because different conservation and rehabilitation plans are required between taxa representing independent lineages (Fig. 4).
Molecular markers are generally selected by taking into account the resolution of the target taxon and the marker to be analyzed. However, there may be differences in resolutions depending on the phylogeny of each taxonomic group. Therefore, it is of primary importance to select useful molecular markers for phylogenetic and phylogeographical analyses. In the case of Isoёtes species of East Asia and North America, which are known to have undergone relative differentiation more recently, it is necessary to analyze the phylogeny and biogeographical histories with molecular markers at high resolutions. Thus far, phylogenetic and biogeographical studies of Isoёtes have been performed using a small number of nuclear and chloroplast DNA sequences (e.g., Hoot et al., 2006; Pereira et al., 2017). The next generation of sequencing (NGS) technology continues to evolve, and analysis costs are becoming lower. Therefore, studies to develop high-resolution molecular markers by comparing nucleotide sequences of whole chloroplast genomes have been active (Park et al., 2016), as have those to elucidate phylogenetic relationships and biogeographic histories (Firetti et al., 2017). However, the entire chloroplast genome of Isoёtes plants is only reported for the North American species I. flaccida (Karol et al., 2010). In order to understand the phylogeny and evolutionary histories of Isoёtes plants distributed around the world, it is necessary to select representative taxa from each clade and analyze the structures and complete nucleotide sequences of the chloroplast genomes using the NGS technique. It is also necessary to understand the precise divergence time and biogeographic history of each clade through a molecular clock analysis (Fig. 5).
The classification system of Isoёtes is based on the surfaces ornamentation of megaspores (Pfeiffer, 1922). Pfeiffer (1922) classified megaspores in Isoёtes into four types, and Hickey (1986) reorganized these further into 12 categories. Although the molecular phylogeny of Isoёtes does not form monophyletic groups according to the shape of each megaspore type (Cox and Hickey, 1984), it would be interesting to determine the evolutionary direction of megaspores. In addition, in Isoёtes, speciation by polyploidization often occurs after hybridization. It is necessary to resolve the controversy as to whether the evolution of megaspores can be interpreted as a result of speciation by hybridization or whether it is associated with the habitat environment. Furthermore, it is necessary to estimate the timing of the evolution of spores.