AbstractThe study aims are to examine the characteristics of artificial and natural hybrids between Viola albida var. albida (= albida, from below) and V. albida var. chaerophylloides (= chaerophylloides, from below), and to confirm if hybrids could be fertile and make populations in their native habitats. The 1st filial (= F1, from below) leaf shape produced by artificial crossing between albida and chaerophylloides was the same as that of V. albida var. takahashii (= takahashii, from below), and F1 bore also both chasmogamous and cleistogamous flowers. F1 seed number was 9.6 per cleistogamous pods, which was remarkably less than the average of 38.2 for albida and chaerophylloides, but the germination rate was all similar. The leaf type of self-crossed 2nd offsprings (= F2, from below) showed all leaf types found in the Viola albida complex, but the ratio of chaerophylloides leaf type was relatively low. Individuals whose F2 leaf type was restored to albida produced an average of 31.4 seeds per capsule, meaning that fertility was restored. On the other hand, individuals of F2 takahashii leaf type come to fruition a low average of 10.4 seeds per capsule, which is similar to that of takahashii. The results of crossbreeding experiment, where is their native habitats, were similar to that of laboratory. Both albida and chaerophylloids in Mt. Bulmyeong distribute extensively, but takahashii make a small population only in places where albida and chaerophylloides grow together. Summarizing the above results is suggesting that the speciation of takahashii was done by hybrid between albida and chaerophylloides, and these have been maintained with relatively small population by cleistogamous capsules.
INTRODUCTIONThe family Violaceae listed up about 1,100 species in 22 genera worldwide (Ballard et al., 2013), and of them about 45 taxa of genus Viola are distributed in Korea (Kim, 1986; Kim et al., 1991; Park, 2009). The Viola albida complex consists of V. albida var. albida, V. albida var. takahashii, and V. albida var. chaerophylloides (Kim et al., 1991; Jang et al., 2006; Ahn, 2015). Taxa within this group have no difference in morphological characteristics such as shape and color of reproductive and vegetative organ, shape and distribution of trichome, pollen ultrastructure, except for leaf shape (Kim, 1986; Jang, 2012). No difference and/or very little difference was also found in experiments of isoenzyme (Kim et al., 1991), random amplified polymorphic DNA (Koo et al., 2010), internal transcribed spacer (ITS) DNA (Whang, 2006), and plastid DNA (Jang et al., 2006). It was further revealed that the genes, VaKN1, VaSTM, VaCUC2, and VaAS1, directly affect the leaf morphology development, and there was absent of nucleotide sequence variation among the species of the complex (Srikanth et al., 2019). However, the expression levels of these genes were different for each plant part and according to developmental stage of the leaf (Srikanth, 2014). The leaves of the taxa within this complex gradually change from simple leaf with regular teeth in the order of V. albida var. albida, V. albida var. takahashii, and V. albida var. chaerophylloides to compound leaves having tri- to pentafoliolate (Kim et al., 1991; Lee, 1999).
There are especially numerous intermediate leaves form between from V. albida var. albida to V. albida var. chaerophylloides (Whang, 2006). Because of these characteristics, it has been speculated so far that V. albida var. takahashii originated from a hybrid of V. albida var. albida and V. albida var. chaerophylloides (Kim, 1986; Kim et al., 1991; Lee, 1999). As ancillary evidence for the theory of the origin of V. albida var. takahahsii by hybrid, there are low pollen fertility, sympatric distribution, and the expectation of free hybridization of taxa within this complex (Kim et al., 1991; Whang, 2006). However, there is no direct experimental evidence for the view that V. albida var. takahashii is hybrids of V. albida var. albida and V. albida var. chaerophylloides so far.
In addition, in taxa of the genus Viola, interspecies hybridization occurs freely, resulting in the appearance of various intermediate types (Russell, 1960), and hybrids of about 28 taxa within the genus Viola have been reported (Hama, 2002). In Korea, several hybrids have been also reported in the native habitate of V. albida var. chaerophylloides, such as V. woosanensis, V. ibukiana, V. × palatina, V. × wansanensis, V. chejuensis, (Yoo and Jang, 2013). In particular, V. × palatina and V. × wansanensis do not bear seeds and/or grow fruit in both chasmogamous and cleistogamous pods (Park, 2012). This is believed to be due to the reduced fertility of hybrids, and they appear only temporarily and are difficult to form populations in nature (Jang, 2012). This is consistent with the results of confirming the sterility of several F1 seeds, including violet × V. albida var. chaerophylloides, obtained through crossbreeding of violets (Lee, 2013). On the other hand, V. albida var. takahashii exist even in places where there are no V. albida var. albida and V. albida var. chaerophylloides nearby, and there is an opinion that its populations are maintained by producing seeds in their native habitat, so it is viewed as an independent taxon rather than a hybrid (Jang, 2012).
The aim of study is, first, to examine the characteristics of hybrids through artificial crossbreeding between V. albida var. albida and V. albida var. chaerophylloides. Second, this study is to verify the probability of speciation of V. albida var. takahashii through hybrids by confirming if hybrids have fertility and can form populations in their native habitats.
MATERIALS AND METHODSPlant materials and artificial crossThe plants within the Viola albida complex used in the breeding experiment were the V. albida var. albida, V. albida var. takahashii, and V. albida var. chaerophylloides. These were collected from the year of 2005 to 2016 at Mt. Naejangsan, Mt. Jeoksangsan, and Mt. Bulmyeongsan of Joellabuk-do. The collected materials were transplanted into the plant cultivation room at Science Hall 1 of Jeonbuk National University and used for artificial breeding. The plants collected from the native habitats were individuals that grew sympatric within 2–5 m in diameter. As shown in Fig. 1, the leaf types of taxa within this complex varied greatly from the simple leaf of V. albida var. albida to the palmate compound leaf of V. albida var. chaerophylloides. Crossbreeding was done using representative individuals of V. albida var. albida (Fig. 1A) and V. albida var. chaerophylloides (Fig. 1J–L) which exhibited the same leaf phenotype over three generations. Before performing artificial crossbreeding, the seeds were first subjected to low-temperature treatment, and then chasmogamous flowers were induced. The pollen was artificially transferred from the stamen to the pistil of the counterpart using a brush, and this was repeated several times for about three days immediately after flowering.
Open pollination in native habitat and plant collectionTaxa within the Viola albida complex intensively live along the 500 m section from the parking lot at the three-way intersection of the entrance to Hwaamsa Temple, Wanju-gun, Jeollabuk-do. Viola albida var. chaerophylloides is growing on the valley side of the hiking trail, and V. albida var. albida is at the bottom of a mountainside. In particular, in the vicinity of north latitude 36o3′53″, east longitude 127o16′57″, both V. albida var. albida and V. albida var. chaerophylloides were growing wild together. Therefore, it was expected that hybridization could easily occur through open pollination. Seeds were collected from the chasmogamous pod of V. albida var. albida and V. albida var. chaerophylloides on 10 May 2014. In addition, three individuals of V. albida var. takahashii, which did not exist in 2013 at the same location, were collected on 3 May 2014 and transplanted to the laboratory.
Seed harvestIf fertilization is successful, the ovary part of the pollinated individual swells within a week, and the seeds gradually mature. Before the outer fruit coat was dried, the fruit was wrapped in a plastic bag (a synthetic fiber pouch with three sides closed in a rectangular shape, 11.5 cm × 9.5 cm) and its entrance part was then tied with a thin wire. When the capsule’s outer coat dries, the seeds splatter in all directions in a pouch. These seeds were placed in an opened Eppendorf tube for drying 2–3 more days, and then had storage at 4oC.
Seed germination and cultivationOne milliliter of distilled water was added to the Eppendorf tube containing the seeds and cold treatment was then performed in a refrigerator at 4oC for one week. Gauze was laid on Petri dish with a diameter of 90 mm, and 8 mL of an aqueous solution of gibberellic acid concentration of 25 mg/L was moistened. Then the cold-treated seed was put on gauze and sealed using parafilm. The concentration of gibberellic acid was referenced based on the experimental results of Kim and Um (1994). Seeds were germinated under two fluorescent lamps of 40 W and 30 W turned on for 24 h in a thermostat (LABTEC, Villmergen, Switzerland) maintained at 15oC and 20oC, respectively. After germination, when cotyledons appeared, they were transferred to a 105-hole plug tray filled with horticultural bed soil. When two to three primary leaves developed, they were transferred to a pot of 90 mm in diameter and then cultivated in the laboratory. When the seedlings had five to seven leaves, cleistogamous seeds were formed, and these were harvested by the method described above. Harvested seeds were also cultivated as described above. Significance verification on the seed germination rate was performed using the SAS program ver. 8 (SAS Institute Inc., Cary, NC, USA).
ITS sequence analysis for verification of artificial cross of F1 offspringTo verify the success of artificial breeding, the genotype was determined using the ITS regions for the first filial (F1) individuals. Among F1 individuals, V. albida var. albida♀ × V. albida var. chaerophylloides♂, V. albida var. albida♂ × V. albida var. chaerophylloides♀, and V. albida var. albida and V. albida var. chaerophylloides, the parents of hybrids, were used as the molecular analysis samples. For the isolation of nucleic acids from plants, the Doyle and Doyle (1987) method was used with some modifications. After DNA was diluted to a concentration of 5 ng/μL, a polymerase chain reaction (PCR) was performed using ITS1 and ITS4 primers (White et al., 1990). The PCR product was subjected to gel electrophoresis, cut a band around 700 bp, and purified with an extraction kit (ELPIS-Biotech Inc., Daejeon, Korea). The nucleotide sequences were determined using the ABI prism 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
RESULTS AND DISCUSSIONCrossing by artificial and open pollination
Seed germination rate: Seeds of three cross-combinations were obtained through artificial and natural crossbreeding. Seven seeds were harvested when V. albida var. albida was the mother and 24 seeds in the case of V. albida var. chaerophylloides. In general, the average number of chasmogamous seeds per fruit of both V. albida var. albida and V. albida var. chaerophylloides was 38.2, while the artificial hybrids were 13.0, which resulted in a rather small number. The cold treatment was done within five to 10 days after harvesting, and the seeds were then placed in a thermostat. As a result, in the case of the mother of V. albida var. albida, seven plants were germinated, while in the case of the mother of V albida var. chaerophylloides 12 individuals. Viola albida var. albida were sympatrically growing together with V. albida var. chaerophylloides within Mt. Bulmyeongsan. Thirty seeds from the chasmogamous of V. albida var. albida mother were harvested in this place, and their germination rate was 100%. However, only two out of 24 seeds, which are presumed to have been produced through cross-pollination between V. albida var. albida♀ and unknown♂, succeeded in germination (Table 1).
The leaf shape phenotype: F1 leaf type obtained by artificial pollination appeared in the same as that of V. albida var. takahashii, which is an intermediate form between the parent V. albida var. albida and V. albida var. chaerophylloides (Figs. 2, 3). Regardless of whether the mother type was V. albida var. albida or V. albida var. chaerophylloides, F1 had the same leaf type. However, as the growth and development progressed, the indentation of the subsequent leaves was slightly more severe than that of the first leaf (Fig. 3B, D). In addition, it was also found that a few leaves of F1, which were produced from the V. albida var. albida mother, showed to have the same leaf type of V. albida var. albida (Table 2). This is presumed to have been formed by self-pollination caused by incompletely removing the pollen grains of V. albida var. albida before artificial cross. The 10 among 30 seeds, produced as a result of open pollination in their native habitat, were of the same leaf type as that of V. albida var. takahashii, although the mother was V. albida var. albida (Fig. 4). These are thought to have crossed with the nearby V. albida var. chaerophylloides. And it is also presumed that self-pollination occurred in the mother itself and/or cross-pollination with other V. albida var. albida in the case of the V. albida var. albida leaf type. The above results showed that leaf type of the hybrids obtained through artificial and open pollination showed a typical V. albida var. takahashii leaf type (Fig. 1D, F). That is, the leaf type of V. albida var. takahashii close to that of both V. albida var. albida (Fig. 1B, C) and V. albida var. chaerophylloides was not observed (Fig. 1G–I). And V. albida var. takahashii, which was not absent in year 2013, were newly discovered in year 2014 within the experimental area of Mt. Bulmyeongsan. Individuals with the leaf type of V. albida var. takahashii were gathered in one place. These are also thought to be hybrids between V. albida var. albida and V. albida var. chaerophylloides, meaning that V. albida var. takahashii can be originated through hybridization of V. albida var. albida and V. albida var. chaerophylloides even in their native places. The leaf shape of V. albida var. takahashii collected from the native place was maintained throughout the cultivation period, although the dentation of its leaves became slightly severe as time passed (Fig. 5C). Seeds of unknown the mother or father, collected in a place of Mt. Bulmyeongsan where V. albida var. albida and V. albida var. chaerophylloides grow sympatrically, have germinated one each, one with leaf type of typical V. albida var. takahashii, and the other V. albida var. takahashii close to V. albida var. chaerophylloides (Fig. 5A, B). In view point from the previous results, it is thought that these individuals are not F1 hybrids of V. albida var. albida and V. albida var. chaerophylloides.
Genotyping for F1 based on ITS DNA analysis: To confirm the success of artificial cross, the genotype of F1 was investigated based on ITS DNA sequencing. Five different cases of genotype were examined (Fig. 6). Viola albida var. albida, V. albida var. chaerophylloides, V. albida var. albida♀ × V. albida var. chaerophylloides♂, V. albida var. albida♂ × V. albida var. chaerophylloides♀, V. albida var. albida seeds naturally hybridized in Mt. Bulmyeongsan between V. albida var. albida♀ and unknown♂, a member of unknown♀ × a member of unknown♂ (e.g., Table 1). They are also referenced previously reported in GenBank, DZ112179, DQ112181, DQ112183, AY928292, AY928293, and AY928290 (Jang et al., 2006; Whang, 2006). In particular, at the 543rd DNA locus, the base of V. albida var. albida was A, and that of V. albida var. chaerophylloides G, V. albida var. albida♀ × V. albida var. chaerophylloides♂ A, V. albida var. albida♂ × V. albida var. chaerophylloides♀ G, and V. albida var. albida seeds naturally hybridized in Mt. Bulmyeongsan between V. albida var. albida♀ and unknown♂ A (Fig. 6). From the chromatogram, the 543rd peak of A and G of V. albida var. albida and V. albida var. chaerophylloides were significantly higher than that of the other experimental groups respectively, and there was also no overlapping (Fig. 6). However, in the other experimental groups, the chromatogram peaks of A and G at the 543rd locus appeared very low, and overlapped (Fig. 6), confirming that these are F1 hybrids between V. albida var. albida and V. albida var. chaerophylloides (Ahn, 2015).
Fertility verification of hybrids
F1 seed formation and its germination rate: All F1 hybrids produced chasmogamous and cleistogamous flowers. Their seeds are continuously produced by cleistogamous flowers until the above-ground part of the plant died. The cleistogamy bore seeds from a minimum of one to a maximum of 25 per pod, and the average number of seeds was rather low with 9.6 (Table 2). This result partly supports the previous report that the pollen fertility of V. albida var. takahashii is low (Kim et al., 1991). The production rate of seed per pod is low because of the low fertility of pollen grains in spite of cleistogamous flowers, which have evolved to make sure self-pollination as well as to increase the number of individuals (Lord, 1979). The average germination rate of F1 most high from artificial pollination in nature at 32.7%, and the next was collected seeds of V. albida var. takahashii at 15.2%, from artificially crossbreeding seeds between V. albida var. albida and V. albida var. chaerophylloides 8.3% in order (Table 2). On the other hand, the average germination rate of the seed of V. albida var. chaerophylloides, treated with gibberellin, was 18.9% (Kim and Um, 1994). The average number of seeds per pod of F1 obtained through the artificial crossing of V. albida var. albida and V. albida var. chaerophylloides was 10.8, and the germination rate was rather low with an average of 8.3%. The number of F1 seeds per pod obtained by open pollination in the native habitat was 8.0. Its germination rate was 32.7%, the smallest amount of seeds, but the highest germination rate. The number of seeds per pod collected V. albida var. takahashii from the native habitat was 10.8, and the germination rate was 15.2% (Table 2). Although the amount of seeds of F1 is smaller than the crossbreeding between V. albida var. albida and V. albida var. chaerophylloides, the germination rate is similar, so it is suggested that some populations in native habitats could be formed.
F2 phenotype: Germination rate and leaf type observation of F2 were used by harvesting cleistogamous seeds formed by self-fertilization of F1 hybrids. The leaf type of F2 was found in all taxa in the Viola albida complex (Fig. 1). However, leaf types of V. albida var. albida and V. takahashii var. chaerophylloides were more numerous than those of V. albida var. albida (Fig. 7). In addition, the leaf type of F2 was not uniformly the same as that of F1 hybrids (Fig. 7A, B), showed numerous intermediate forms between V. albida var. albida and V. albida var. chaerophylloides (Fig. 7E–L). Individuals (Fig. 7E, F) that appeared to be intermediate between V. albida var. albida and V. albida var. takahashii had very irregular and deep lobes at leaf margin. However, they could be grouped into the V. albida var. albida leaf type in light of the morphological distinctions that the leaf primordium starts from the apical meristem and gradually develops into mature leaves (Choi and Whang, 2019). On the other hand, typical V. albida var. takahashii (Fig. 1C, D) developed a few lobes and were symmetrical around the central vein. The leaf type of V. albida var. takahashii (Fig. 1E, F), which is close to V. albida var. chaerophylloides, had the deepest lobes resembling a compound leaf.
In a single individual of F1 hybrid mother, F2 leaf developed in both V. albida var. albida and V. albida var. takahashii leaf type at the same time (Fig. 8). As described above, F2 leaves showed the same trend in regardless F2 seeds from (1) artificial cross, (2) open pollination, (3) collection of V. albida var. takahashii, etc. This phenomenon was consistent with the report that V. albida var. takahashii exhibited numerous leaf types in its native habitat (Yoo et al., 2013). Kim et al. (1991) estimated that the leaf polymorphism within this group was due to the strong ability to fruit by cleistogamous and vegetative propagation by rhizome. In addition, it was also considered to be due to the reproduction mechanism of simultaneous cross-fertilization and self-fertilization through chasmogamous and cleistogamous flowers (Jang et al., 2006). In this study, it was confirmed that cleistogamy alone could be probable to have polymorphism of V. albida var. takahashii leaf type.
Whether F2 seed production or notF2 also bore cleistogamous seeds like F1 hybrids (Table 3). The average number of seeds per capsule was 19.8. Among them, the average seed production, in the case of the same leaf type of V. albida var. albida in F2 was 31.4, but 10.4 in the case of V. albida var. takahashii leaf type. That is, the seed production of the F2 leaf type of V. albida var. albida was about three times higher than that of V. albida var. takahashii. Comparison of the number of seeds F2 of V. albida var. albida and V. albida var. takahashii showed a statistical difference as a result of the t-test (Table 3). The pods of F2 of V. albida var. albida leaf shape bore more than 30 seeds respectively. However, the leaf type of F2 of V. albida var. takahashii was over 30 in only one out of a total of 46 harvests, and then the rest were remarkably low (Ahn, 2015). Some individuals whose F2 was returned to V. albida var. albida have also seemed to have recovered fertility. This means that as the generation of hybrids are repeated, the proportion of both V. albida var. takahashii within the population of hybrids and that of descendants of hybrids will decrease. In particular, this phenomenon will be further profound if hybrids produce offspring only through self-fertilization within cleistogamous flower. Two taxa, V. albida var. albida and V. albida var. chaerophylloides, are actually widely distributed in one of their native places, Mt. Bulmyeongsan. However, V. albida var. takahashii is formed in small-scale populations only where two taxa, V. albida var. albida and V. albida var. chaerophylloides, coexisted. This is considered to be the same as the phenomenon in which V. albida var. takahashii are found relatively rarer than V. albida var. albida and V. albida var. charerophylloides (Park, 2012). In summary of above results, V. albida var. takahashii were originated through hybridization between V. albdia var. albida and V. albida var. chaerophylloides, and it is also considered that its population maintenance is possible through self-fertilization by both cleistogamy and vegetative propagation of rhizome. However, compared to V. albida var. albida and V. albida var. chaerophylloides, it has lower seed production capacity, so it is expected to maintain the population at a smaller or lower density than the others within the Viola albida complex in its native habitat.
ACKNOWLEDGMENTSThis study was partly supported by a research grant of Jeonbuk National University (2022–2023).
Fig. 1.Leaf polymorphism within the Viola albida complex. A. V. albida var. albida. B, C. intermediate between V. albida var. albida and V. albida var. takahashii. D. V. albida var. takahashii. E–G. Intermediate forms between V. albida var. takahashii and V. albida var. chaerophylloides. H–J. V. albida var. chaerophyloides. 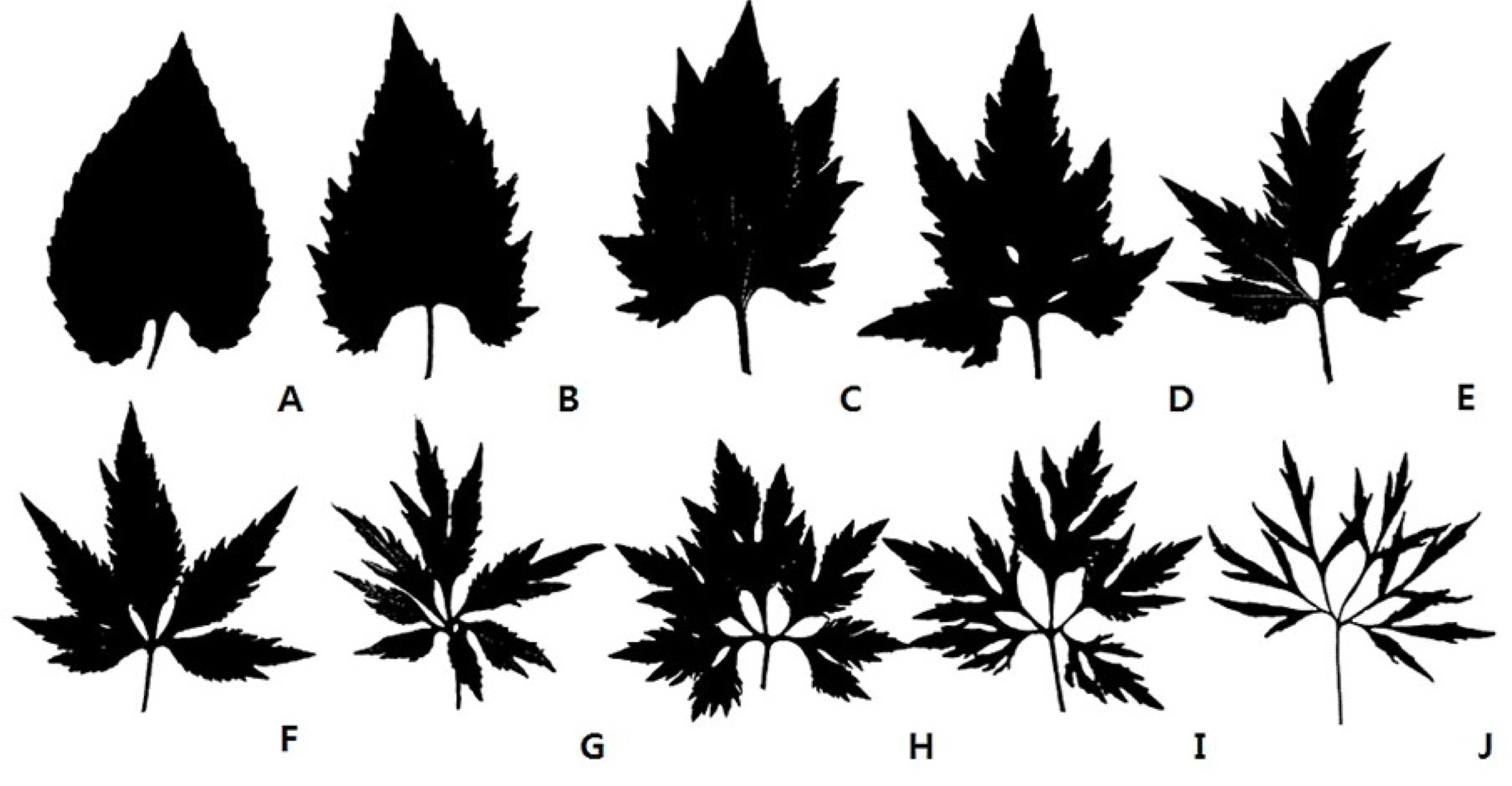
Fig. 2.Violets used for artificial cross. A. V. albida var. chaerophylloides. B. V. albida var. albida. Scale bar = 10 cm. 
Fig. 3.Leaf shape of F1 hybrids obtained by reciprocal crosses; one is between V. albida var. albida♀ and V. albida var. chaerophylloides♂, and the other V. albida var. chaerophylloides♀ and V. albida var. albida♂. A, C: in two months after germination. B, D: in three months after germination. Scale bar = 1 cm. 
Fig. 4.The V. albida var. takahashii leaf shape obtained by the germination used for the seeds that collected in chasmogamic flower of V. albida var. albida. A–C should be naturally hybridized V. albida var. chaerophylloides at Mt. Bulmyeong, Wanju-gun, Jeonbuk. Scale bar = 1 cm. 
Fig. 5.Leaf shape from seeds assumed as formed by natural hybridization between V. albdia var. albida and V. albida var. chaerophylloides at Mt. Bulmyeong. A is close to V. albida var. albida from V. albida var. takahashii, whereas B is V. albida var. chaerophyllodies. C from the seed of V. albida var. takahashii collected in Mt. Bulmyeong. Scale bar = 1 cm. 
Fig. 6.Chromatogram showing nucleotide sequences of the region of position 543 of ITS DNA. Va: V. albida var. albida, Vc: V. albida var. chaerophylloides, NT1: V. albida var. albida♀ × V. albida var. chaerophylloides♂, NT2: V. albida var. albida♂ × V. albida var. chaerophylloides♀, TN11: Natural hybrid between V. albida var. albida♀ and unknown species at Mt. Bulmyeong. 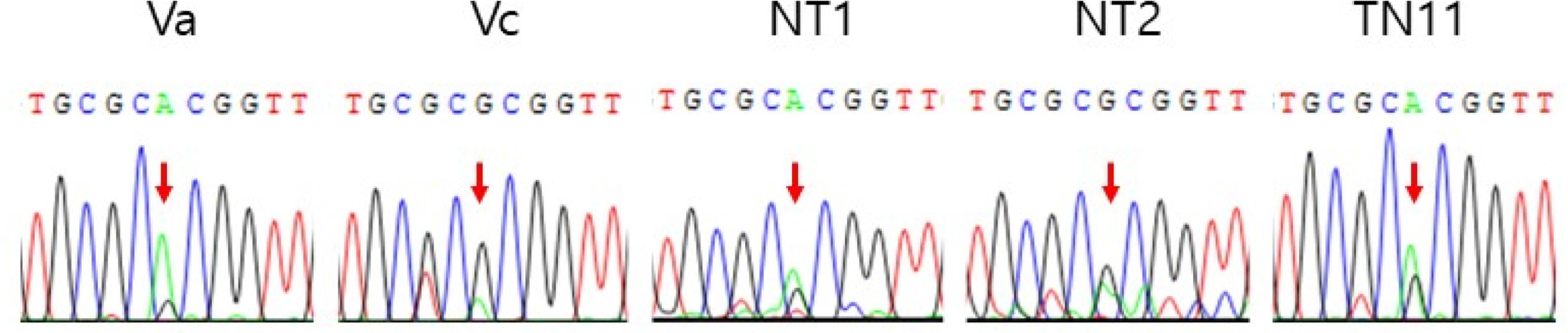
Fig. 7.Leaf shape obtained after hybridization between V. albida var. albida and V. albida var. chaerophylloides. A–D. F1, E–L. F2. E–F: close to V. albida var. albida. G–I. close to V. albida var. takahashii. J–L. close to V. albida var. chaerophylloides. Scale bar = 1 cm. 
Fig. 8.Leaf shape of V. albida var. takahashii collected from Mt. Bulmyeong (A) and its offspring (B–H). Scale bar = 1 cm. 
Table 1.Germination rate and leaf shape of the 1st offspring of both artificial and natural hybrids. Table 2.Number of the F1 seed per pod and their germination ratio.
LITERATURE CITEDAhn, SH. 2015. A speciation study of Viola albida complex based on artificial hybridization. MS thesis,. Jeonbuk National University, Jeonju, Korea. 24 pp.
Ballard, HE. de Paula-Souz, J and Wahlert, GA. 2013. Violaceae. The Families and Genera of Vascular Plants. XI: Kubitzki, K (ed.), Springer, Berlin. 298-326.
Choi, YK and Whang, SS. 2019. A comparative study of early leaf development in the Viola albida complex. Korean Journal of Plant Taxonomy 49: 1-7.
Doyle, JJ and Doyle, JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11-15.
Hama, E. 2002. Viola of Japan. Seibundo Shinkosha Publishing Co, Tokyo.
Jang, S-K. 2012. Phylogenetic study of Viola section Pinnatae Wang. Ph.D. dissertation. Kangwon National University; Chuncheon, Korea. 199.
Jang, S-K. Lee, W-T and Yoo, K-O. 2006. Taxonomic study on Viola albida var. albida and its related taxa. Korean Journal of Plant Taxonomy 36: 163-187.
Kim, KS. 1986. Studies of comparative morphology on the Korean Viola species. Ph.D. dissertation. Sungkyunkwan University; Suwon, Korea. 105.
Kim, KS. Sun, BY. Whang, SS and Chung, GH. 1991. Biosystematic study on the genus Viola in Korea: Comparative morphology of the Viola albida complex. Korean Journal of Botany 34: 229-238.
Kim, G-T and Um, T-W. 1994. Effects of gibberelic acid treatment on seed germination of several Viola species. Applied Ecology Research 8: 74-77.
Koo, JC. Tak, HJ and Whang, SS. 2010. Taxonomic study of Viola albida complex based on RAPD data. Korean Journal of Plant Taxonomy 40: 118-129.
Lee, CB. 1999. Illustrated Flora of Korea. Hyangmunsa, Seoul. 791 pp.
Lee, CH. 2013. Exploitation of Korean native ornamental plants for new crops and investigation of their commercial feasibility: In vitro propagation and breeding of native flowers and ornamental trees. Report for 21C Frontier Research of Ministry of Science and ICT. Chungbuk National University, Cheongju. 323 pp.
Lord, EM. 1979. Physiological controls on the production of cleistogamous and chasmogamous flowers in Lamium amplexicaule L. Annals of Botany 44: 757-766.
Park, CW. 2009. The Genera of Vascular Plants of Korea. Academy Publishing Co, Seoul. 393 pp.
Park, S-C. 2012. Violets of Korea. Hamkkeganeun-gil, Seoul. 264 pp.
Russell, NH. 1960. Studies in the photoperiodic responses of violets (Viola). The Southwestern Naturalist 5: 177-186.
Srikanth, K. 2014. Differential expression of certain key genes mediates leaf morphology variation in Viola albida complex. Ph.D. dissertation,. Jeonbuk National University; Jeonju, Korea. 153.
Srikanth, K.. Hill, RS and Whang, SS. 2019. A correlation between leaf shape and its related key genes in Viola albida complex. In Vitro Cellular & Developmental Biology – Plant 55: 409-420.
Whang, SS. 2006. Analysis of ITS DNA sequences of the Viola albida complex. Korean Journal of Plant Resources 19: 628-633.
White, TJ. Bruns, T. Lee, S and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications. Innis, MA. Gelfand, D. H.b. Sninsky, JJ. White, TJ (eds.), Academic Press, San Diego, CA. 315-322.
Yoo, K-O and Jang, S-K. 2013. Korean Peninsula Violet Looking at Features. Gi-sung-sa Co,
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||