INTRODUCTION
It is known that the Flora of Korea consists of about 4,500 species of vascular plants (Lee, 2003). Among them, about 570 species of endemic plants which are native to Korea were reported (Paik, 1999). Nakai (1952) defined 11 endemic genera to the Korean peninsula, but according to the current classification, six or seven genera were recognized as endemic (Nakai, 1952; Lee, 1969; Paik, 1999; Kim, 2004; Park, 2007; Chung et al., 2017) (Table 1). Mankyua established new genus of Pteridophyte in 2001 as Jeju-gosari-sam (Sun et al., 2001). Echinosophora was known as an endemic genus by Nakai (1952), but this genus was recognized as an endemic species of Sophora according to Lee et al. (2004), and excluded from the endemic genus. Therefore, there are six genera consisting of: Mankyua, Abeliophyllum, Coreanomecon, Hanabusaya, Megaleranthis, and Pentactina as defined by the Flora of Korea Editorial Committee (Park, 2007). In present study, the category of the Korean endemic genus of angiosperm was confirmed based on information in the Flora of Korea (Park, 2007).
Seed morphology has been shown to provide useful characteristics for the analysis of taxonomic relationships in angiosperms (Corner, 1976; Shetler and Morin, 1986; Takhtajan, 1991). Additionally, the details of the sculpturing of the seed coat can be quite variable and of systematic importance. At the genus level, seed morphology has been very useful in distinguishing both species groups and occasionally in distinguishing closely related species or species complexes (Lester, 1991; Knapp and Helgason, 1997). Moreover, ovule orientation is very important for the seed developmental process of genus or family levels (Tobe, 1989; Bouman and Boesewinkel, 1991). In addition, seed appendages, such as aril, caruncle, operculum, wing, and hairs are very useful to confirming the taxonomic relationships (Kapil and Bouman, 1980). Therefore, seed morphological study is very important to a taxonomist.
In the 21st century, the era of biodiversity and biological sovereignty, the plant resources of the country are becoming more important as intellectual property sources. Among them, seed germplasm resources, which occupy most of them, can be evaluated as having high value (Kim, 2021). Therefore, the seed development and seed coat characteristics of five Korean endemic genera are presented here. The basic information of ovule and seed could help to prepare for the era of intellectual property rights wars on seed genetic resources in the future.
MATERIALS AND METHODS
This study investigated the ovule development and seed coat composition of five endemic genera recognized among angiosperms native to Korea (Table 1). The investigated taxa are presented with material collection information (Table 2). Except for Pentactina, all materials were obtained from natural populations by the corresponding author directly. The experimental method was basically to section the young ovule in transverse section to check the orientation of the ovule, the development and type of the integument, the thickness of the integument, the development of appendages of the mature seed, the type of the seed coat, and the characteristics of the endosperm. For the experiment, inflorescences and seeds were collected from young to mature seed stages before flowering for each taxon, fixed with F.A.A., dehydrated with an ethanol series, embedded with Technovit 7100 resin solution, trimmed and sectioned to a thickness of 5 μm with a microtome (Leica RM 2235, Heidelberg, Germany). The sections were stained with Toluidine Blue O for 5 minutes, decolorized in running water and then dried. The dried slides were then mounted with Entellan solution. Photos were taken with a camera (Olympus DP 70, Tokyo, Japan) attached to the microscope. For scanning electron microscope (SEM) observation, mature seeds were dehydrated using critical point drying. After drying, the mature seeds were coated with platinum using an ion sputter (Hitachi E1010, Tokyo, Japan) and observed by SEM (Carl Zeiss, Supra 55VP, Germany). The terminology of seeds and seed coats was described according to the terminology of Schmid (1986). Furthermore, we referred to seed characteristics from the Seed Atlas of Korea (Korea National Arboretum, 2017). The description was the alphabetical order according to the genus name.
RESULTS AND DISCUSSION
Abeliophyllum distichum Nakai (Miseon-namu)
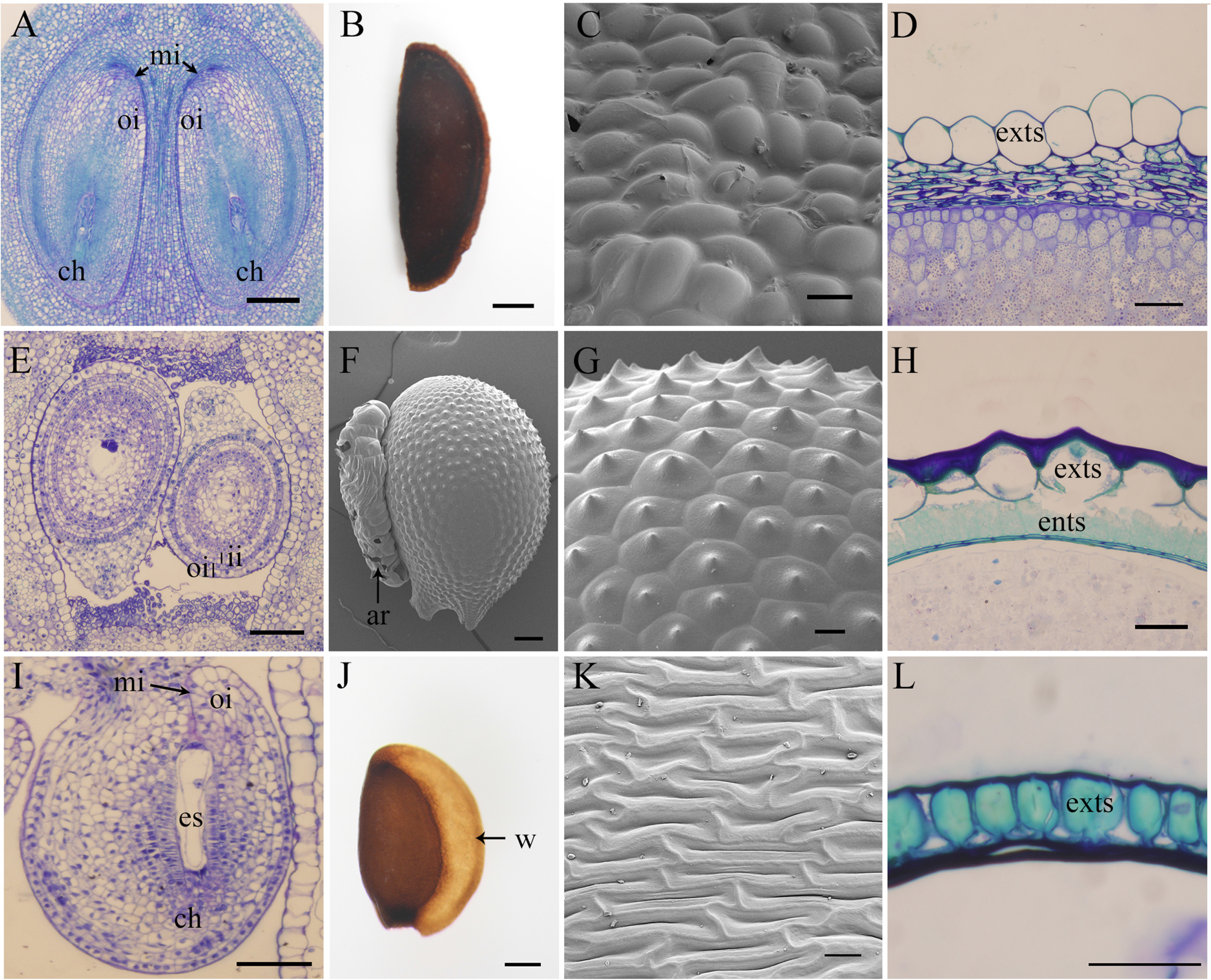
Abeliophyllum distichum belongs to the Oleaceae and bisexual flowers. Flowers are characterized by flower buds of the previous year and bloom before leaves appear in early spring. Several varieties have been developed as ornamental resources. The carpel is syncarpous, mature fruits are indehiscent. The shape of fruit is described as fan-shaped, two carpels each on the left and right, and one carpel is developed on one ovule. The frequency of mature seeds is that in some cases, both ovules are mature, and in other cases, only one of the two ovules is mature. The size of the carpel is approximately 10 mm elongated. The ovule is anatropous, and the unitegmic develops into an outer integument (Fig. 1A, Table 3). The thickness of the outer integument is composed of 9 to 10 cell layers, and these are developed into multiple layers at the mature stage. In the mature seed stage, the exotegument expands colliculate like a balloon and functions to protect the embryo and endosperm. Therefore, the seed coat type of Abeliophyllum is an exotestal. The color of mature seeds is dark brown, and has an ellipsoid shape of approximately 7.6–9.7 × 2.5–4.3 mm. The surface of the seed is slightly colliculate (Fig. 1A–D, Table 3). The thickness of the mature seed coat is 170–180 μm and the thickness of the exotesta is 90–100 μm (Table 3).
It is reported that Abeliophyllum is close to Fontanesia and Forsythia (Lee and Park, 1982; Kim et al., 2000). Forsythia has multiple ovules in the center of the carpel and capsule, different from Abeliophyllum, whereas seed coat characteristics are very similar to Abeliophyllum with roundish thick walled exotesta (Ghimire and Heo, 2014), and have no crystals in the petioles as a shared characteristic (Song and Hong, 2012). In addition, while Fontanesia have a solitary pendulous ovule and small roundish samara similar to Abeliophyllum, the seed characteristics are different from Abeliophyllum (Ghimire and Heo, 2014). Therefore, it thought that the status as an endemic genus is supported by the fan-shaped samara.
Coreanomecon hylomeconoides Nakai (Maemi-kkoch)
Coreanomecon belongs to the Papaveraceae and has bisexual flowers. The gynoecium of the Coreanomecon is monocarpic. The dehiscent form of the mature fruit is a capsule, dorsal and ventral striae are dehisced together. The size of the carpel is approximately 5 mm × 2 mm. Many ovules develop above and below the dorsal and ventral sides of the carpel. The frequency of seed maturation is extremely high. The ovule is an anatropous, with the bitegmic ovule developing into an outer integument and inner integument (Table 3). The thickness of the outer integument is composed of 2 to 3 cell layers, and the inner integument also develops into 2 to 3 cell layers. There is no multiplication of integuments during seed maturation. At this time, the exotesta and endotestal cells develop into sclerenchyma cells and function to protect the embryo and endosperm. Therefore, the type of seed coat development of Coreanomecon was confirmed as an exo-endotestal type (Fig. 1E–H). The color of the seed is light brown and has an elliptical shape with a length of approximately 1.5–1.9 × 1.1–1.4 mm. In particular, the seed surface was supported as an endemic genus along with the pollen characters as it was observed that small echinate projections were developed (Fig. 1F). An aril was also developed in the funicle side of the mature seed (Fig. 1F, Table 3). The thickness of the mature seed coat is 100–120 μm and the thickness of exotesta is 50–60 μm (Table 3).
In the wild, it is often confused with Hylomecon, but its inflorescence pattern is terminal, distinguished from Hylomecon which has axillary inflorescences. Hylomecon has rhizomes, tricolpate pollen grain, and 2n = 24 in chromosome number. On the other hand, Coreanomecon has taproots, 12-pericolpate pollen grain, and 2n = 12 in chromosome number (Lee and Kim, 1984; Kim et al., 1999). In addition, in the mature seed surface characteristics, Coreanomecon has echinate, whereas Hylomecon has a reticulate seed surface, which is clearly distinguished (Ghimire et al., 2019). Even in the phylogenetic tree analyzed by rbcL, matK, and ITS data, Coreanomecon was not clustered in the Hylomecon or Chelidonium clades but kept an independent clade (Yun and Oh, 2018; Ghimire et al., 2019). Therefore, it is supported as an endemic genus.
Coreanomecon grows wild in Mts. Baekunsan of Gwangyang city and Jirisan in southern part of Korea, and it seems that the extinction crisis has been overcome through restoration projects in government or private botanical gardens. Incidentally, it has reached the level of prevalence that it can be purchased from flower nurseries in the market by propagation through seed germination.
Hanabusaya asiatica Nakai (Keumgang cholong-kkoch)
Hanabusaya belongs to Campanulaceae and its flowers are bisexual sympetalous. The gynoecium is a tri-syncarpous and each carpel has countless ovules attached as axil placentation. The frequency of seed maturation is exceedingly high, and many mature seeds are developed. The ovule is small anatropous and unitegmic (Table 3). The integument is composed of 7 to 8 layers in its young stage, and it develops into a structure in which the exotestal of 2 to 3 cell layers acts as a mechanical protection during seed maturation. Therefore, the seed coat type is exotestal. The seed size is approximately 1.5–1.7 × 0.8–1.0 mm. The color of seed is brown. Seed wing appendages developed with the flatten at the opposite side of raphe bundle and appeared to develop an oval shape, such as Codonopsis which has a seed wing (Fig. 1I–L, Table 3) (Korea National Arboretum, 2017). The thickness of the mature seed coat is 20–25 μm and the thickness of exotesta is 14–15 μm (Table 3).
Hanabusaya are native to the subalpine region of Mts. Seoraksan, Daeamsan, Whaaksan, and Myongjisan, in the northern part of the Korean peninsula and show their beauty with their unique purple flower color (Kang et al., 2015; Chang et al., 2016). The ovary of Hanabusaya has three carpels, syncarpous, and countless ovules are developed for each carpel, which eventually develops into many mature seeds. In addition, this flower has a unique character developing flower disk. Although Hanabusaya was published as a new genus with the characteristics of anther fusion and cauline leaves gathering on the upper part of the stem (Nakai, 1911), Lee et al. (1986) have reported that Hanabusaya is very similar to the Campanula in pollen morphology. Therefore, the separation into the endemic genus should be reconsidered. In this study, the seed and seed coat anatomical characteristics were also indicated similar to the Campanula and Codonopsis with wing and seed surface sculpture (Shetler and Morin, 1986; Korea National Arboretum, 2017). Therefore, it should be reconsidered in taxonomical treatment as an endemic genus. It may need more evidence to confirm monotypic status of this genus. On the other hand, Hanabusaya asiatica was evaluated as an endangered (EN) species by International Union for Conservation of Nature (IUCN) criteria evaluation (Chang et al., 2016).
Megaleranthis saniculifolia Ohwi (Modemi-pul)
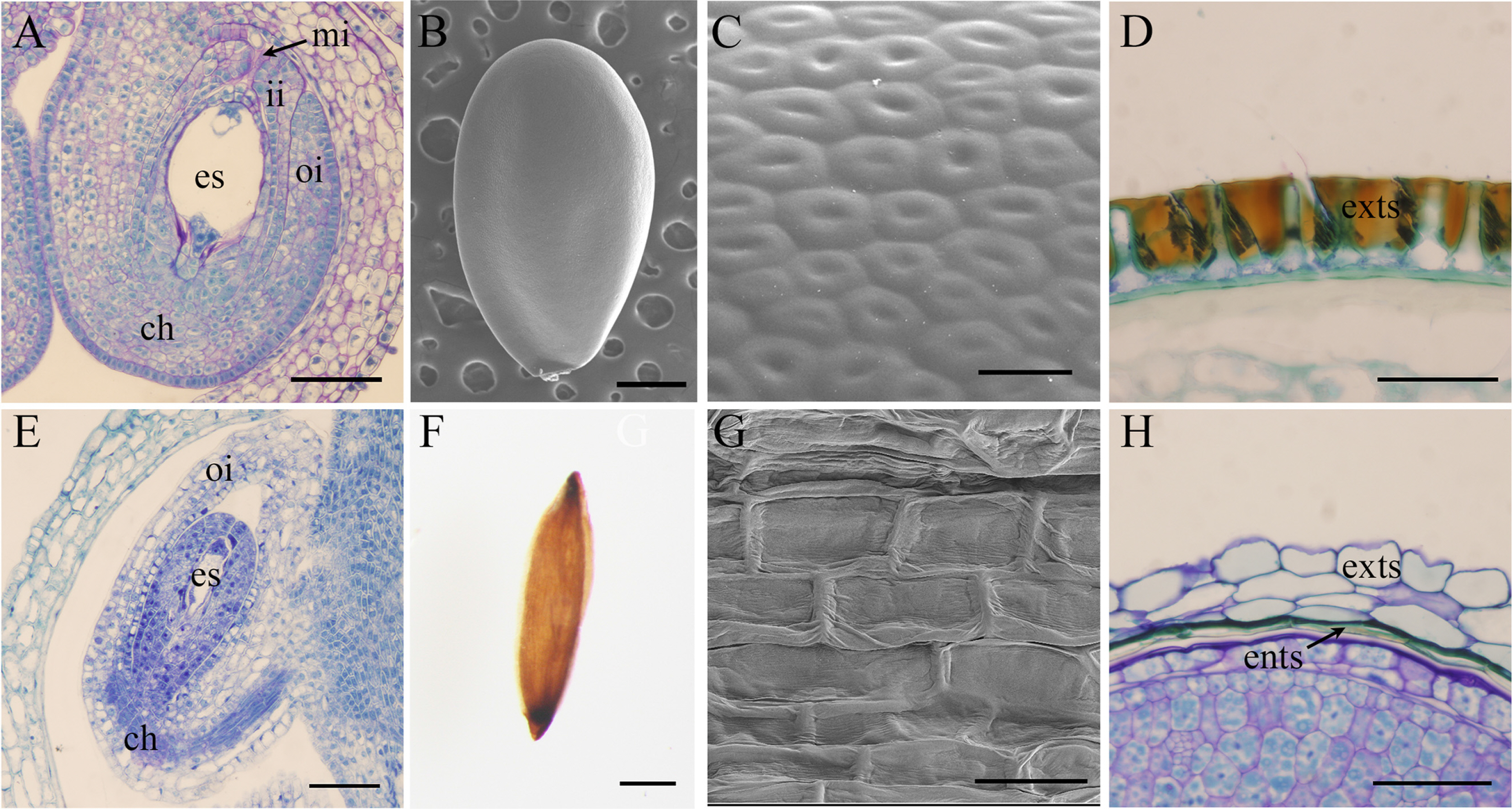
Megaleranthis belongs to Ranunculaceae and has bisexual flowers. The carpel is apocarpous with 5 to 7 carpels irregularly. The type of mature fruit is follicle with loculicidal dehiscence. The size of the carpel was approximately 8 mm × 3 mm. Several ovules are developed side by side at the end of ventral side in one carpel. The frequency of seed maturation is extremely high. The ovule is an anatropous, and with the bitegmic ovule developing into an outer integument and inner integument (Table 3). Several ovules develop in an up and down fashion on the ventral side of the carpel. The outer integument is composed of 4 to 5 cell layers, and the inner integument develops into 2 to 3 cell layers. In the mature seed, the exotesta of the seed coat developed a macrosclerenchyma cell layer with tannin and functions to protect embryo and endosperm. Therefore, the seed coat type of Megaleranthis is exotestal. The color of seed is black. The shape of the seed is an ellipsoid approximately 1.6–1.9 × 1.0– 1.2 mm. Seed appendages are not developed (Fig. 2A–D, Table 3). The thickness of the mature seed coat is 40–50 μm and the thickness of exotesta is 35–40 μm (Table 3).
Megaleranthis was first collected from the Unbong region in Mt. Jirisan and reported as a new genus by Ohwi (Ohwi, 1935). Although there are researchers who claim that it is taxonomically incorporated into the Trollius, so far it is recognized as an endemic genus according to the differences in the vegetative characteristics such as the absence of cauline leaf and interstitial growth of the receptacles after flowering (Kim and Lee, 1987; Tamura, 1995). However, in the seed characteristics, there was no specific difference from Trollius (Jang and Heo, 2005; Heo and Suh, 2008; Jung and Heo, 2017). In addition, the pollen morphology also indicated a similarity to Trollius, thus the status as endemic genus was not recognized, rather they insisted that Megaleranthis should be combined as Trollius chosenensis (Lee, 1990). To confirm taxonomic status of Megaleranthis the further study like molecular phylogeny is necessary. On the other hand, Megaleranthis saniculifolia was evaluated as an endangered (EN) species by IUCN criteria evaluation (Chang et al., 2016).
Pentactina rupicola Nakai (Keumgang-ingamok)
The gynoecium of Pentactina is pentamerous and apocarpous carpel. The type of mature fruit is follicle with loculicidal dehiscence. The size of carpel is approximately 1.5–1.6 × 0.6–0.8 mm. The frequency of seed maturation does not seem to be high. This is because the development time of microspore and megaspore does not match even though the reproductive organs of the Pentactina are bisexual. This is considered to be due to the phenomenon of protandry in order to induce out cross-pollination (K. Heo, pers. obs.). The ovule is anatropous and unitegmic (Fig. 2E, Table 3). Two ovules developed in the ventral side of each carpel. The type of placenta is a marginal placentation (Fig. 2E). The thickness of the integument is composed of 3 to 4 cell layers. In the mature seed stage after double fertilization, the endotesta accumulates as a tannin substance and functions to protect the embryo and endosperm. Therefore, the development type of the seed coat is confirmed as endotestal type (Fig. 2E–H). The color of the mature seed is brown with a fusiform shape approximately 1.1–1.3 × 0.3–0.4 mm. Seed appendages are not developed (Fig. 2F). Seed surface sculpture is reticulate in all examined vouchers (E & SNU) (Fig. 2G, Table 3). The thickness of the mature seed coat is 40–50 μm and the thickness of the exotesta is 15–20 μm (Table 3).
In general, bitegmic ovule is dominant in Rosaceae. Unitegmic ovule is already reported in diverse genera within Rosaceae, such as Adenostoma, Alchemilla, Aphanes, Aruncus, Geum, Holodiscus, Neviusia, Potentila, Rhodotypos, Rubus, Sibiraea, and Ulmaria (Davis, 1966), suggesting that the unitegmic ovule must have been secondarily derived in the family. In this study, we confirm the presence of unitegmic ovule in Pentactina and Spiraea. Therefore, all genera of tribe Spiraeeae (Potter et al., 2007; Zhang et al., 2017) that have been examined (Aruncus, Holodiscus, Pentactina, Sibiraea, and Spiraea) show unitegmic ovules. It is likely that unitegmic ovules should have evolved multiple times within Rosaceae and that the unitegmic ovule may be a synapomorphy for tribe Spiraeeae. Moreover, unitegmic members have an unspecialized and non-multiplicative in the seed coat characteristics (Johri et al., 1992). However, Pentactina has an endotestal seed coat type and is regarded as an autapomorphy in the Rosaceae.
Pentactina is an important endemic genus whose native range is limited to Mt. Keumgangsan, north of Gangwon province in the Korean peninsula (Chang et al., 2016). In a politically divided country, it is very difficult to secure plant materials for Pentactina. Therefore, in this study, seed materials were obtained by collecting carpels from the 1930s specimen kept in the Herbarium of the School of Biological Sciences, Seoul National University and the Royal Botanic Garden Edinburgh Herbarium (E). A closely related plant is known as Spiraea (Song et al., 2020). Song et al. (2020) obtained seed material from specimens at the Herbarium of Royal Botanic Garden Edinburgh and investigated the characteristics of the seed surface. The results of the study argued that the seed surface characteristics of striate-rugose as an endemic genus because it is different from the closely related taxon, Spiraea (Song et al., 2020). In addition, molecular data supported it as an endemic genus of the Korean peninsula (Lee and Hong, 2011). However, as a result of SEM observation of the seed in this study, seed surface characteristics of Pentactina is a reticulate shape similar to that of the tribe Spiraeeae. Therefore, it could not support the endemic genus of Pentactina by the seed surface characteristics. In case of the achene, it is difficult to separate the seed from the fruit wall, but in case of the follicle, the seeds are easily to separate from the fruit wall. It is judged that no error will be made in observing the surface of the seed and fruit, if the materials are carefully collected. Therefore, we think that this problem can be clearly resolved through the observation of the pericarp structure in the future. On the other hand, Pentactina rupicola was evaluated as a critically endangered (CR) species by IUCN criteria evaluation (Chang et al., 2016).