INTRODUCTION
Bhutan is located in the biodiverse Himalayan region and is part of both the Palearctic and Indo-Malayan biogeographic realms. It has a variety of climates, ranging from subtropical to temperate to alpine, and houses an array of flora and fauna (Myers et al., 2000; Banerjee and Bandopadhyay, 2016). Much was learned about the botanical landscape during the Flora of Bhutan project which began in 1975, with detailed collections documented by Grierson and Long (1983, 1984, 1987, 1991, 1999, 2001) and Noltie (1994, 2000). This expedition had established 4523 species of angiosperm belonging to 1,416 genera and 266 families, while 22 gymnosperm species were recorded, containing 15 genera and eight families (Y. Dorji, 2010). However, these records underestimate the actual diversity since several unknown areas were not explored by botanists from the time of Griffith (1838) to Grierson and Long (2001). Additionally, certain species such as Litsea chartacea Hook. f., Canarium strictum Roxb., and Lippia alba (Mill.) N. E. Br. ex Britton & P. Wilson, which are expected to be found in similar climate as those in Assam, Darjeeling, Sikkim, Arunachal Pradesh in India, and Tibet in China were accounted for in the Flora of Bhutan. This Flora of Bhutan led to the description of many new species and created the largest floral database for Bhutan between 1983 and 2001.
In recent years, botanical excursions have seen improvements due to increase access to sites. Not only have new species been described but several new records also have been reported for Bhutan. From 2009 to 2022, 19 species were described by multiple authors (Fryer and Hylmo, 2009; Kadota, 2010; Lidén, 2010; Ohba and Akiyama, 2010; Podlech, 2010; Rushforth, 2010; Sukhorukov, 2012; Wen and Shi, 2012; Yoshida and Grey-Wilson, 2012; N. Gyeltshen et al., 2017, 2020; Yoshida et al., 2017a, 2017b; C. Gyeltshen et al., 2019; P. Gyeltshen et al., 2022). In 2009, two species were described: Cotoneaster hicksii J. Fryer & B. Hylmö and C. bumthangensis J. Fryer & B. Hylmö. The following year, five more new species were added to the lists: Aconitum bhutanobulbilliferum Kadota, Prunus harae H. Ohba & S. Akiyama, Astragalus paroensis Podlech, Dactylicapnos platycarpa Lidén, and Griffitharia karchungii (Rushforth) Rushforth. In 2012, three more new species were discovered— Meconopsis bhutanica Tosh. Yoshida & Grey-Wilson, Dysphania bhutanica Sukhor, and Prunus gongshanensis J. Wen. Four years later in 2016 only one species was discovered: Meconopsis elongata Tosh. Yoshida, Yangzom & D. G. Long. Three more species Meconopsis gakyidiana Tosh. Yoshida, Yangzom & D. G. Long, Meconopsis merakensis Tosh. Yoshida, Yangzom & D. G. Long and Roscoea megalantha Tosh. Yoshida & Yangzom were identified as new species along with Spathoglottis jetsuniae Gyeltshen, Tobgyel & Dalström, a terrestrial orchid. Chiloschista gelephuense C. Gyeltshen & Dalström was discovered near the hot springs in Gelephu (C. Gyentshen et al., 2019). Two new orchid species were reported by N. Gyeltshen et al. (2020); they were Chiloschista densiflora Gyeltshen, C. Gyeltshen & Dalström and C. himalaica Tobgay, C. Gyeltshen & Dalström. The most recent discovery and addition to the Flora of Bhutan is Begonia menchunaensis P. Gyeltshen & M. Hughes (P. Gyeltshen et al., 2022).
In the past few years, plant enthusiasts have been set out to explore areas that have not been covered before, resulting in the establishment of several new records. This collective effort led to the discovery of 41 species and two genera from 28 different families in Bhutan, which have been added to the Flora of Bhutan between 2019 and 2022 by multiple authors (Rabgay and Kumar 2019; Gurung et al., 2020; Gyeltshen and Dema, 2020; R. Dorji et al., 2021, 2022a, 2022b, 2022c; P. Gyeltshen et al., 2021, 2022; Phuentsho et al. 2021; Rabgay et al., 2021; Tenzin et al., 2021). Table 1 presents the details of the authors who reported new records of plants.
The scientific documentation of new records or discoveries in Bhutan has been rapidly accumulated in recent years. There are several cases where the new plant specimen has been collected and doposited in herbaria, however, these materials remained unpublished. Generally, discoveries and records are reported in local media or kept within individual collections. To unveil these practices, it is essential to publish their findings on a platform that will provide the basis for future management studies. This article attempts to present new records of plant species distribution that have not been previously published in peer-reviewed articles and the Flora of Bhutan. This article includes a brief morphometric description of species, presented as an annotated checklist along with their ethnobotanical uses.
MATERIALS AND METHODS
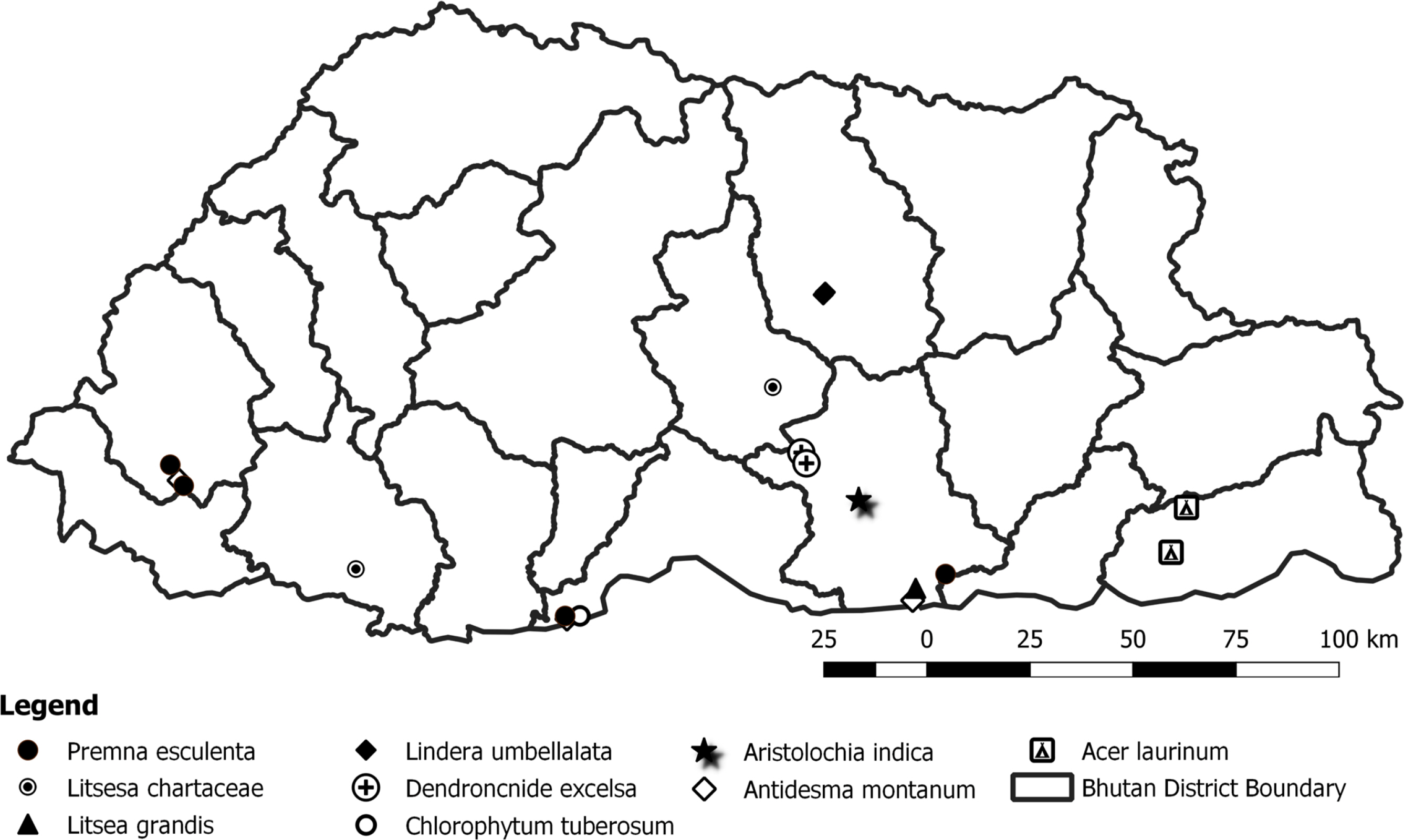
An opportunistic survey was employed while collecting the plant specimens and photographs from the natural habitats of the tropical, subtropical, and temperate belts of Bhutan. Collection of herbarium specimens followed specific protocols, and plants were pressed. The spatial location details were recorded for each species (Fig. 1). The species are labeled and are now maintained at Ugyen Wangchuck Institute for Forest Research and Training (UWIFoRT), Lamai Goenpa Bumthang. New species record was determined mainly from the original description of each species along with illustrated identification keys. Furthermore, to confirm the species identification, vigorous reviews were conducted using the Flora of Bhutan, peer-reviewed literature from elsewhere, and consultation with experts from the region. Additionally, various digital type specimen collections and photographs available in Royal Botanic Garden, JSTOR Global Plant, and Global Biodiversity Information Facility (GBIF) were referenced.
RESULTS AND DISCUSSION
We report here nine species of angiosperm plants that are new to the flora of Bhutan. Four of these species were found in tropical biomes along the southern boarder, Assam and the West Bengal region of India while four others were discovered in warm-broadleaf forests in the central region. Only one species was recorded from the temperate forest. Although these species were already being used by local communities for ethnobotanical purposes, their existence has not been documented in botanical expeditions before. Our thorough examination of literature and expert consultation elsewhere in Himalayan regions validates new records and lists these species as new additions to Bhutan’s flora. Amongst the nine new records, four species of tree were from Lauraceae, Phyllanthaceae, Sapindaceae, and Utricaceae. Three species of shrubs were from Lamiaceae and Lauraceae, and two species of herbs were from the families Aristolochiaceae and Asparagaceae.
Aristolochiaceae
1. Aristolochia indica L., Sp. Pl. 960, 1753 (Fig. 2A).— TYPE: INDIA. Bengal, 1820, N. Wallich, 2704 (K001116840!).
Aristolochia pandurata Jacq., Pl. Rar. Hort. Schoenbr. 4: 49. t. 497, 1804.
Aristolochia lanceolata Wight, Icon. Pl. Ind. Orient. [Wight] v. t., 1858.
Aristolochia maysorensis Fisch. ex Duch., DC. Prod. 15: 479, 1864.
Aristolochia indica is a shrubby perennial climber with a woody rootstock (Kanjilal et al., 2009). Branches are slender, long, glabrous, and grooved. Leaves are fiddle-shaped to linear, alternate and simple 3.5–8 × 2–3.5 cm, ovatelanceolate, entire and petiolate, base truncate, apex acute to acuminate, glabrous; 3–5-nerved at base; petiole 2.5 cm long. Flowers are pale green on the outer surface with a rim of the mouth dark purple color, usually fetid in odor, solitary with white tubular with axillary racemes. Axillary raceme with perianth up to 4 cm long, having inflated pale green glabrum (Das et al., 2010). Flowers bloom from June to October. Fruits are globose, dehiscent, and oblong. Seeds are many with 4–6 mm across, broadly deltoid, flat, and winged all around.
With the addition of A. indica, Bhutan now has six species of Aristolochia (A. griffithaii Hook. f. & Thomson ex Duch., A. nakaoi F. Meak., A. cathcartii Hook. f., A. platanifolia (Klotzsch) Duch., A. tagala Cham synonymized to A. acuminata Lam) (Grierson and Long, 1984). This species can be differentiated by its presence of herbaceous vine and narrow leaves while others species possess heart-shaped leaves (A. griffithii, A. nakaoi, A. cathcartii) and palmately lobed leaves (A. platanifolia).
Specimen examined: BHUTAN. Zhemgang District, Trong, Praling, along the footpath from Praling to Duenmangtshachhu (hot spring), after crossing a small stream and before reaching the first concrete resting place, 27.043691N, 90.808778E, elev. 690 m, 26 May 2022, Tshering TT006 (UWIFoRT).
Distribution: Andaman Islands, Bangladesh, native to India (Assam, Bengal) Myanmar, Nepal, Sri Lanka, Vietnam.
Ethnobotanical uses: Aristolochia indica has long been used as a traditional medicine to treat various disorders. The constituent of this species has been used for medicinal purposes to treat colorea, fever, ulcers, snake bites, leprosy, bowel troubles, diarrhea, and cure skin disease (Jain and De Filipps, 1991; Pezzuto et al., 1998; Krishnaraju et al., 2005; Kanjilal et al., 2009; Dey and De, 2011).
Asparagaceae
2. Chlorophytum tuberosum (Roxb.) Baker., J. Linn. Soc., Bot. 15: 332, 1876 (Fig. 2B).—TYPE: NEPAL. Sunsari District, elev. 230 m, 29 Apr 1974, A. P. Sing & M. B., Chhetry, 17952 (KATH000684!).
Chlorophytum tuberosum is a herb grown in tropical biomes. It is about 20 cm tall with gregarious clumps. Leaves strap-shaped all arising from the base 15–30 cm long. The white flower blooms in monsoon (June–July). Petals elliptic, 6 erects stamens in yellow anthers. The fruits have three-edged capsules. There are three species (C. khasianum Hook. f., C. nepalense, and C. arundinaceum Baker) reported in the Flora of Bhutan which are members of the Chlorophytum genus (Noltie, 1994). With the C. tuberosum reported here, four species of the genus Chlorophytum are added to the Flora of Bhutan. This species is closely related to C. khasianum (accepted synonym C. nepalense (Lindl.) Baker) by having long narrow leaves and basal flowers but differs in the shape and size of the tubers and color of flowers. Latter species have larger and elongated tubers when compared and their flowers are yellowish-green in color.
Specimen examined: BHUTAN. Sarpang District, Singye, Phipsoo, along the foot trail and bank of the stream about 2.6 km before reaching Phipsoo Range Office or Army Out Post from Themba, 26.753669N, 90105782E, elev. 206 m, 10 Jan 2021, Tshering TT001 (UWIFoRT).
Distribution: The native range of this species is Nigeria to Eritrea and Tanzania, Central & South India to Myanmar.
Ethnobotanical uses: The dried bulbs and leaves are pounded into flour for bread. Roots have been used as an aphrodisiac in ayurvedic medicine and a lotion is extracted from the tuber and used to treat guinea-worm (Patil and Deokule, 2010).
Lamiaceae
3. Premna esculenta Roxb., Hort Bengal. 46, 1814 (Fig. 2C).—TYPE: INDIA. Buxa Bengal, 2 Feb 1979, J. S. Gamble 6648 (holotype: K000882625!).
Gumira esculenta (Roxb.) Kuntze, Revis. Gen. Pl. (1891).
Premna esculenta is a shrub with hairless short-stemmed, branches slender. Leaves simple, decussate-opposite, deciduous, base acute, margin crenate, apex acuminate, dark green, generally glabrous above while slightly paler yellowish pubescent beneath when young. Inflorescence composed of 4–8 opposite cymes, pubescent, quadrangular peduncles 1–2 cm long, bracts linear ca. 3–4 mm long. Flowers bisexual is borne in leaf axils with greenish-yellow or cream white, pedicels ca. 3 mm long, calyx campanulate 4–5 lobed, corolla funnel-shaped, 4 subequal lobed, densely pubescent at the throat, didynamous, epipetalous, filament glabrous and filiform, anthers versatile with 2 locules. Fruits drupaceous, obovoid about 3 mm in diameter, purple, and smooth. Flowers in February to April. Of the eight species of Premna (P. interruptaWall. ex Schauer, P. bracteataWall. ex C. B. Clarke, P. scandens Roxb., P. bengalensis C. B. Clarke, P. barbata Wall. ex Schauer, P. mollissima Roth, P. lucidula Kurz accepted synonyms P. serratifolia L., P. herbacea Roxb.) reported by Grierson and Long (1999) in the Flora of Bhutan, P. herbacea and P. lucidula were reported from elsewhere Darjeeling, Sikkim and Assam in India. Including P. esculenta, seven species established their occurrences in Bhutan under the genus Premna. Often P. esculenta is confused with P. serratifolia however, the latter can be differentiated by longer and narrower leaves typically serrated along the edges.
Specimens examined: BHUTAN. Sarpang District, Singye, Phipsoo, about 500 m upstream of Army Out Post, 26.754347N, 90.104606E, elev. 206 m, 10 Jan 2021, Tshering, TT003 (UWIFoRT); Zhemgang District, Ngangla, Yumdang, 26.864325N 91.019734E, elev. 89 m, 21 Apr 2023; Haa District, Rangtse, along the highway connecting Samtse and Haa, 27.078660N 89.170416E, elev. 826 m, 7 May 2023 and at Gakiling near Amochhu bridge, 27.129368N 89.137799E, elev. 771 m, 5 May 2023, Tshering (UWIFoRT).
Distribution: India (Andhra Pradesh, Assam, Manipur, Mizoram, Tamil Nadu, Tripura, West Bengal), Bangladesh, Myanmar, Thailand.
Ethnobotanical uses: The ethanolic extract from this plant has potential compounds for the treatment of liver, cardiovascular and inflammatory disorders. Traditionally, the people of Bangladesh utilize it for a variety of ethnopharmacological purposes including the treatment of gouts, arthritis, wound healing, colds, cough, headache, jaundice, lipoma, and edema (Mahmud et al., 2012).
Lauraceae
4. Lindera umbellata Thunb., Fl. Jap. (Thunberg) 145, t. 21, 1784 (Fig. 2D)); Benzoin umbellatum (Thunb.) Kuntze, Revis. Gen. 2: 569, 1891.—TYPE: JAPAN. Honshu, 29 Apr 1914, E. H. Wilson, 6591 (K000815629).
Benzoin thunbergii Siebold & Zucc., Abh. Math.-Phys. Cl. Königl. Bayer. Akad. Wiss 4(3): 204, 1846; Lindera thunbergii (Siebold & Zucc.) Makino., Bot. Mag. (Tokyo) 14: 184, 1900.
Lindera hypoglauca Maxim., Bull. Acad. Imp. Sci. Saint-Pétersbourg 12: 71, 1868; Benzoin hypoglaucum (Maxim.) Rehder., Standard Cycl. Hort. 1: 487, 1914.
Lindera obtusa Franch. & Sav., Enum. Pl. Jap. 2: 483, 1878; Benzoin obtusum (Franch. & Sav.) Kuntze., Revis. Gen. Pl. 2: 569, 1891.
Lindera umbellata is a deciduous shrub spicebush. Height 2 to 6 m, bark color green on young, and grey on mature trees. The leaf is hairless dark green, typically narrow-oblong to ovate-oblong at the top, acuminate, with leaf length 5–10 cm, width 2–5 cm, and pinnately veined. Flowers and leaf development occur March–April at the same time. In spring usually, six-petaled flowers are pale yellow-green, and translucent. The leaf appears yellow and small ripen fruit to black in September–October. Including the record of L. umbellata, Bhutan has a record of 10 species with the genus Lindera (L. heterophylla Meisn, L. neesiana Kurz, L. pulcherrima (Nees) Benth. ex Hook. f., L. melastomacea Fern.-Vill., L. bootanica Meisn., L. assamica (Meisn.) Kurz, L. latifolia Hook. f., L. nacusua (D. Don) Merr. and L. hamiltonii Kosterm). Amongst these, one of the closest species to L. umbellata is L. neesiana as both species share morphological similarities such as deciduous growth habits, branching patterns, and fruit characters, however, the former has more ovate to elliptic leaves while the latter has more oblong to lanceolate.
Distribution: Japan, China, United Kingdom.
Specimen examined: BHUTAN. Bumthang District, Chhoekhor, Lamai Goenpa, above and below farm road heading to Tongkhang and old forest road and foot trail that led to Tharpaling Monastery, 27.541511N, 90.722844E, elev. 2,800 m, 12 Dec 2022, Tshering TT005 (UWIFoRT).
Ethnobotanical uses: It possesses aromatic components in its twigs and leaves. The entire tree has a volatile aroma because it contains terpineol and linalool. In Japan, branches have been used for indoor decorations and to produce shavings for aromatic baths. Oil extracted from leaves and fruits contain terpene, alcohol esters, and linalool are the most abundant and have been used traditionally as a medicine for neuralgia, stiff neck, and back pain. The fruit, branches, and leaves are also used to make tea (Maeda et al., 2011).
5. Litsea chartacea (Wall. ex Nees) Hook. f., Fl. Brit. India [J. D. Hooker] 5(13): 170, 1886 (Fig. 2E); Tetranthera chartacea Wall. ex Nees., Pl. Asiat. Rar. (Wallich). 2: 67, 1831; Malapoenna chartacea (Wall. ex Nees) Kuntze., Revis. Gen. Pl. 2: 572, 1891.—TYPE: INDIA. Sikkim, Rishap, elev. 1,676 m, Oct 1877, G.King 5162 (K000357516!).
Litsea chartacea is an evergreen tree up to 8 m in height. Usually, twigs are dark, often reddish brown, and slightly ridged. Leaves elliptic, 8–16 × 2.5–6.5 cm, base cuneate, apex acute to acuminate, underside glabrous, and minutely pubescent. Petioles 1–1.5 cm, umbel buds 1–5 with 4–6 mm in diameter densely arranged. Male umbels 5–7 flowers, pedicles ca. 1 mm, sepal oblong, 2.5 mm, stamen 4–9 mm. Inflorescences 1.5 cm long with up to 4 fruits, peduncles 12 mm, pedicles 15 mm. Flowers from May-June, and fruits from October (Flora of Nepal, 2023). This species is more closely allied to Litsea laeta (Nees) Trimen, but comparatively the leaves of L. chartacea are oval-shaped while the leaves of L. laeta are elliptical. The record of this species ascertains its distribution in Bhutan which was projected in the Flora of Bhutan (Grierson and Long, 1984).
Distribution: India (Arunachal Pradesh, Assam, Meghalaya, and Sikkim), Nepal, Yunnan in China, and Vietnam (Michael, 2023).
Specimens examined: BHUTAN. Trongsa District, Langthel, Khoshala, below and above the highway connecting Trongsa and Zhemgang District, 27.317512N, 90.600423E, elev. 1,530 m, 27 Nov 2022, Tshering TT008 (UWIFoRT); Zhemgang District, Dakphai, grown along with Schima wallichii (DC.) above and below the highway; at Khomshar, grown along with Alnus nepalensis D.Don; Chhukha District, Arekha, Sinchekha, 26.876627N 89.588415E, elev. 1246 m, Tshering (UWIFoRT). Generally, this species occurs in a warm-broadleaf forest.
Ethnobotanical uses: There are no specific ethnobotanical uses of this species documented. However, in general Litsea species are quite popular for essential oils steamed from the ripped fruits. The oil has incredibly effective cleansing properties to the skin with soothing aroma.
6. Litsea grandis (Nees) Hook. f., Fl. Brit. India [J. D. Hooker] 5(13): 162, 1886 (Fig. 2F); Polyadenia grandis Nees, Pl. Asiat. Rar. (Wallich). 2: 62, 1831; Tetranthera grandis (Nees) Wall. ex Meisn., Prodr. 15: 188, 1864; Malapoenna grandis (Nees) Kuntze, Revis. Gen. Pl. 2: 572, 1891.—TYPE: PHILIPINES. Gatubig River, Samar, Feb 2016, M. Sablaya, 107 (K000815245!).
Litsea grandis is a mid-canopy tree up to 34 m tall with about 80 cm in diameter. Leaves simple, alternate, pinnately veined with dense hairs, sometimes whitish. Flowers white-yellow ca.14 mm in diameter. Fruits ca. 8 mm usually red placed on enlarged flower capsules. Found in undisturbed tropical biome forests up to an elevation of 800 m, usually grown on hillsides and ridges with sandy to clay soil. There are 12 species belonging to the genus Litsea (L. sericea (Wall. ex Nees) Hook. f., L. kingii Hook. f., L. cubeba (Lour.) Pers., L. salicifolia (Roxb. ex Nees) Hook. f., L. laeta (Nees) Trimen, L. elongata (Nees) Hook. f., L. monopetala (Roxb.) Pers., L. nervosa (Meisn.) Grierson & D. G. Long., L. glutinosa (Lour.) C. B. Rob., L. panamanja (Buch.-Ham. ex Nees) Hook. f., L. albescens (Hook. f.) D. G. Long, L. nitida (Roxb. ex Nees) Hook. f.) in Bhutan (Grierson and Long, 1984), and now with two additional species, the number has grown up to 15 species. L. grandis is closely related to L. glutinosa in terms of leaves, flowers, colors, and fruits. They can be differentiated by slightly thicker and leathery leaves than those of L. glutinosa. In addition, the leaf margin of L. glutinosa is typically flat while the other is slightly rolled under.
Specimen examined: BHUTAN. Zhemgang District, Panbang to Mathanguri at the bank of Mangdechhu and Drangmechhu river confluences, usually grown on sandy soil, 26.830401N, 90.947198E, elev. 122 m, Tshering, TT001 (UWIFoRT).
Distribution: Indo-China, Burma, Thailand, Peninsular Malaysia, Sumatra, Java, Lesser Sunda Islands, Borneo (throughout the island), Philippines, Celebes, Moluccas, New Guinea.
Ethnobotanical uses: Though it has an unpleasant smell it is used for planks, furniture, and carvings. Oil extracted from the seed is used to make hair cream.
Phyllanthaceae
7. Antidesma montanum Blume, Bijdr. Fl. Ned. Ind. 17: 1124, 1827 (Fig. 3A).—TYPE: CHINA. Kwangtung Province, 7 Aug 1887, C. Ford 258 (Holotype: K000061454!).
Antidesma leptocladum Tul., Ann. Sci. Nat., Bot. Sér. 3, 15: 199, 1851.
Antidesma menasu (Tul.) Müll. Arg., DC. Prodr. 15: 257, 1866.
Myrica darrisii H. Lév., Repert. Spec. Nov. Regni Veg. 12: 537, 1913.
Antidesma discolor Airy Shaw, Addit. Ser. 8: 212, 1980.
Antidesma montanum is commonly known as a mountain currant tree. Leaves simple, alternate, distichous, stipules in pairs, linear-lanceolate, pubescent, petiole 0.25–0.7 (–1.2) cm long, lamina 8–22 × 2.5–7.7 cm, usually oblong to elliptic or oblanceolate, apex gradually acuminate with mucronate tip, base acute to rounded or cuneate, margin entire, chartaceous, glabrous except on midrib; secondary nerves prominent beneath, 5–9 pairs, ascending; tertiary nerves broadly reticulate. Inflorescence axillary or terminal raceme, flowers unisexual, dioecious, clusters of flowers in males distant than in females. Fruits drupe, elliptic, somewhat oblique, turning red. Including the present new record of A. montanum, three species of the genus Antidema (A. acidum Retz., A. acuminatum Wight) are locally known from Bhutan (Grierson and Long, 1987). Both A. montanum and A. acidum share similar habitats, leaves, flowers, and fruits; nevertheless, the former can be distinguished by a leathery leaf texture, and purple or red fruits, whereas the latter has thinner, more delicate leaves and greenish-yellow fruits.
Distribution: Native to Andaman Island., India (Assam), Bangladesh, Borneo, Cambodia, China, Jawa, Laos, Lesser Sunda Island, Malaya, Maluku, Myanmar, Nansei-Shoto, New Guinea, Nicobar Island, Philippines, Australia, Sri Lanka, Sulawesi, Sumatera, Taiwan, Thailand, Tibet, Vietnam.
Specimens examined: BHUTAN. Sarpang District, Singye, Phipsoo, 10 Jan 2021, 26.753669N, 90105782E, elev. 206 m, Tshering, TT004 (UWIFoRT); Haa District, Sombaykha, Rangtseney, 27.0915242N 89.15937139E, elev. 751 m; Zhemgang District, Manas, Hatilaura, 26.800535N 90.939831E, elev. 150 m, Tshering (UWIFoRT).
Ethnobotanical uses: Fruits and leaves are utilized locally for food and traditional medicine. The leaf extracts are applied for diabetic therapy, anti-inflammatory effect to treat eye diseases and relieve chest pain. It is also used against headaches, and thrush in children, removing kidney stones, and skin disease. Tea from the leaves is used as a tonic for mothers after giving birth and applied to ulcers and pains. The roots are used to treat measles, chickenpox, malaria, and stomach ache.
Sapindaceae
8. Acer laurinum Hassk. Tijdschr. Natuurl. Gesch. Physiol. 10: 138, 1843 (Fig. 3B).—TYPE: THAILAND. Dai Kar (Intanon) Pah Ageam Nath peak, elev. 2,070 m, H. B. G Garrett 77 (Holotype: K000640927!).
Acer cassiifolium Blume, Rumphia, 3: 193, t. 167. B, f. 2, 1849.
Acer curranii Merr, Philipp. J. Sci. C 4: 285, 1909.
Acer garrettii Craib, Bull. Misc. Inform. Kew 1920(9): 301, 1920.
Acer decandrum Merr,, Lingnan Sci. J. 11: 47, 1932.
Acer chionophyllum Merr, Brittonia 4: 109, 1941.
Acer jingdongense T. Z. Hsu, Acta Phytotax. Sin., 21: 339, 1983.
Acer laurinum is a tall deciduous tree that may grow up to 48 m tall, with grey bark and green to glaucous or purple glabrous branchlets (Irwanto and Irsyam, 2021). Leaves simple, oblong to elliptic or lanceolate, upper surface dark glossy green, lower surface glaucous, white or light bluish grey, five to six lateral veins on each side of the midrib, margins entire or remotely serrate, apex acuminate; petiole 1.75–10 cm long glabrous green (Irwanto and Irsyam, 2021). The inflorescence is lateral and corymbose or paniculate with unisexual flowers; the peduncle is 5–35 mm long, and the pedicels are 4–17 mm long and 2.5–10 cm long. Flowers yellowish, 5-merous, staminate flowers on separate plants; sepals ovate, 0.2–0.3 cm long, petals shorter, stamens 8–12, inserted into the nectar disc. Calyx 5, free, overlapping in the bud, 2.5–3 m long. Corolla 5, free, white, 1.5–2.5 mm long. Stamens 4–8 whorl, connected on the disk; filament 5 mm long in male flowers, and 2.2 mm long in female flowers, white, glabrous; anther 0.75 mm long; mericarps 2, 8–13 mm long, ovate; wings erect, 4–7 × 1–2.5 cm, asymmetrically obovate, hairy (Bloembergen, 1948). Prior to this discovery of A. laurinum, Bhutan was known to have 11 species (A. Oblongum Wall. ex DC, A. hookeri Miq., A. sikkimense Miq., A. stachyophyllum Hiern, A. taronense Hand.-Mazz., A. thomsonii Miq., A. cappadocicum Gled., A. campbellii Hook. f. & Thomson ex Hiern, A. caudatum Wall., A. pectinatum Wall. ex D. Don and A. sterculiaceum Wall.) of the genus Acer (Grierson and Long. 1991).
Specimen examined: BHUTAN. Samdrupjongkhar District, Gomdar, Serchenmo, grown at the place called Tabgangyoe with associated trees Michelina champacca L., Beilschmiedia Nees., 91.567106N, 26.917322E, elev. 1,662 m, 21 Dec 2022; Gomdar, Bazorphu, 27.025914N, 91.604356E, elev. 1,824 m, Dorji et al., D001 (UWIFoRT).
Distribution: Myanmar, Borneo Combodia, China, India, Indonasia, Laos, Malaysia, Philippines, Thailand, Vietnam, Nepal, Singapore, Sumatra, Java, Timur, Sulawesi (Chan et al., 2020; Irwanto and Irsyam, 2021).
Ethnobotanical uses: A tree is one of the integral raw materials for the construction of houses and furniture in the localities of Assam and Indonesia (Hakim and Miyakawa, 2013), which is consistent with the uses found in Bhutan.
Urticaceae
9. Dendrocnide excelsa (Wedd.) Chew., Gard. Bull. Singapore, 21: 203, 1965 (Fig. 3C); Urera excelsa Wedd., Ann. Sci. Nat. Bot. sér. 4. 1: 178, 1854.—TYPE: AUSTRALIA. New South Wales, May 1824, A. Cunningham s.n. (K000675699!).
Urera rotundifolia Wedd., Ann. Sci. Nat. Bot. sér. 4, 1: 177, 1854.
Laportea gigas Wedd., Monog. Urtic. 129, t. 3, 1856;
Urticastrum gigas (Wedd.) Kuntze., Rev. Gen. Pl. 2: 635, 1891.
Dendrocnide excelsa commonly known as a giant stinging tree is an endemic plant to Eastern Australia, usually found in the rainforest. It is a medium to large-size tree sometimes up to 40 m in height with fluted/flanged trunks (Floyd, 1989).
Leaves are simple, alternate, and margins regularly toothed to almost entire with dull green lamina broad-ovate, usually 10–25 cm long, 7–20 cm wide. Deeply cordate at the base often with the two lobes overlapping, usually covered with firm stinging hairs eaten by insects and mammals. Inflorescences unisexual, panicles to 12 cm long. Achene ca. 2 mm long, warty, pedicel to 5 mm long, and very fleshy. Yellow-green flowers appear from November to April while the matured purple or blackish nut attached to a whitish stalk is apparent from March-August. In Bhutan, Dendrocnide sinuta (Blume) Chew is the only species belonging to the genus Dendrocnide that has been documented (Grierson, and Long, 1983). D. excelsa can be distinguished by its larger, heart-shaped leaves, as opposed to D. sinuta which has smaller and more elongated leaves.
Distribution: Australia, Southeast Asia, and Northeast India.
Specimen examined: BHUTAN. Zhemgang District, Trong, Bermo, about 3.5 km upstream of Tingtibe at Botanical Park, both above and below the highway, 27.159187N, 90.669469E, elev. 575 m, 20 Nov 2022, Tshering TT007 (UWIFoRT).
Ethnobotanical uses: Dendrocnide excelsa endemic to Australia is known as a giant stinging tree (Floyd, 1989) and is also a concern for the locality due to its constituent of venomous hairs. Although stinging hairs cause an intense persistent sting, the flesh is edible after removing the stinging hairs. The sting of this plant when comes into contact with skin, hairs penetrate through soft tissues causing severe tingling sensation which can last sometimes for 24 hours or even months. It may be possible to relieve pain by wetting exuded water of its own and pulling off the sting.